| General Information of Drug Metabolite (DM) (ID: DM006746) |
| DM Name |
BAY-1002670 M4
|
| Structure |
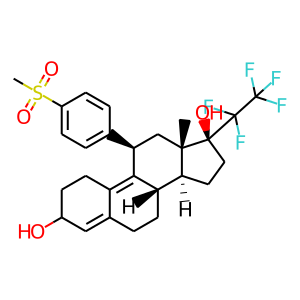
|
|
3D MOL is unavailable
|
2D MOL is unavailable
|
|
|
|
|
|
|
|
| Full List of Drug-Metabolizing Enzyme (DME) Related to This DM |
| DME(s) Producing This DM through Metabolism |
| DME Name |
DME ID |
Reactant |
Reaction |
Related Drug |
REF |
|
Succinic semialdehyde reductase (AKR7A2)
|
DME0234
|
| Reduction - Reduction |
BAY-1002670
|
[1] |
|
|
|
|
|
|
|
| DME(s) Metabolizing This DM |
| DME Name |
DME ID |
Product |
Reaction |
Related Drug |
REF |
|
Cytochrome P450 3A4 (CYP3A4)
|
DME0001
|
| Oxidation - Oxidationn |
BAY-1002670
|
[1] |
|
Cytochrome P450 3A4 (CYP3A4)
|
DME0001
|
| Oxidation - Oxidationn |
BAY-1002670
|
[1] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Full List of Drug(s) That Produce This DM By Metabolism |
| BAY-1002670 |
DR1831
|
Phase 2 |
Uterine leiomyoma |
|
|
| References |
| 1 |
Characterization of the pharmacokinetics of vilaprisan: bioavailability, excretion, biotransformation, and drug-drug interaction potential. Clin Pharmacokinet. 2018 Aug;57(8):1001-1015.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


