| General Information of Drug (ID:
DR0013) |
| Drug Name |
Methoxyamphetamine beta
|
| Synonyms |
Beta-methoxyamphetamine; p-Methoxyamphetamine; para-methoxyamphetamine; paramethoxyamphetamine; (+-)-p-Methoxyamphetamine; 1-(4-Methoxybenzyl)ethylamine; 1-(4-methoxyphenyl)propan-2-amine; 1-p-Methoxyphenyl-2-aminopropane; 1-p-Methoxyphenyl-2-propylamine; 2-(4-Methoxy-phenyl)-1-methyl-ethylamine; 2-Amino-1-(4'-methoxyphenyl)propane; 23239-32-9; 4-Methoxyamphetamine; 50505-80-1; 64-13-1; CHEMBL278663; DEA No. 7411; DL-p-Methoxy-alpha-methylphenethylamine; NSC 32757; p-Methoxy-alpha-methylphenethylamine
|
| Indication |
Discovery agent
|
Investigative
|
[1]
|
| Structure |
|
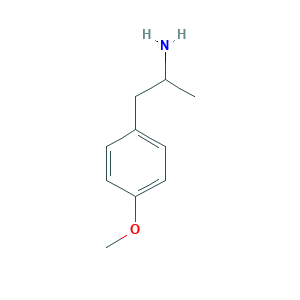 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
165.23 |
Topological Polar Surface Area |
35.2 |
| Heavy Atom Count |
12 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 31721
- CAS Number
-
- TTD Drug ID
- D0R7ZC
- Formula
- C10H15NO
- Canonical SMILES
- CC(CC1=CC=C(C=C1)OC)N
- InChI
- 1S/C10H15NO/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3
- InChIKey
- NEGYEDYHPHMHGK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


