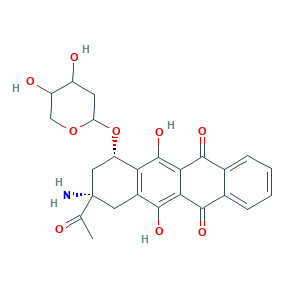
| Synonyms |
Amrubicin [INN]; LS-183761; SCHEMBL119022; (+-)-(7S,9S)-9-Acetyl-9-amino-7-((2-deoxy-beta-D-erythro-pentopyranosyl)oxy)-7,8,9,10-tetrahydro-6,11-dihydroxy-5,12-naphthacenedione; (7S,9S)-9-acetyl-9-amino-7-(4,5-dihydroxyoxan-2-yl)oxy-6,11-dihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-9-acetyl-9-amino-7-(4,5-dihydroxytetrahydropyran-2-yl)oxy-6,11-dihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; 110267-81-7; AC1L4374; AN-16624; BC677604; FT-0662134
|
| Cross-matching ID |
- PubChem CID
- 178149
- PubChem SID
-
33501458
; 50067579
; 78309456
; 104426973
; 126671522
; 142026085
; 164835807
; 223654684
; 226490852
; 245323584
; 249582551
; 252214726
- CAS Number
-
- TTD Drug ID
- D04BEN
- Formula
- C25H25NO9
- Canonical SMILES
- CC(=O)C1(CC(C2=C(C1)C(=C3C(=C2O)C(=O)C4=CC=CC=C4C3=O)O)OC5CC(C(CO5)O)O)N
- InChI
- 1S/C25H25NO9/c1-10(27)25(26)7-13-18(16(8-25)35-17-6-14(28)15(29)9-34-17)24(33)20-19(23(13)32)21(30)11-4-2-3-5-12(11)22(20)31/h2-5,14-17,28-29,32-33H,6-9,26H2,1H3/t14?,15?,16-,17?,25-/m0/s1
- InChIKey
- VJZITPJGSQKZMX-HUVCIAIMSA-N
|