| Cross-matching ID |
- PubChem CID
- 54746
- PubChem SID
-
10387
; 7848050
; 7978840
; 8183775
; 12013713
; 14759961
; 14808642
; 34718715
; 46508571
; 48415679
; 49965419
; 53787199
; 57313654
; 75968790
; 85788035
; 92309005
; 92713133
; 92740959
; 93166933
; 103771070
; 104305757
; 117539013
; 118047283
; 126624984
; 126656189
; 126684133
; 134337565
; 135013805
; 135652674
; 135989394
; 137001464
; 142175117
; 144206142
; 152101006
; 160963596
; 162179026
; 163133315
; 164155372
; 164824353
; 170464667
; 175267048
; 176484246
; 179151225
; 184546096
; 196111573
; 223682255
; 223820795
; 226427091
; 252222293
; 252391240
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04EGX
- Formula
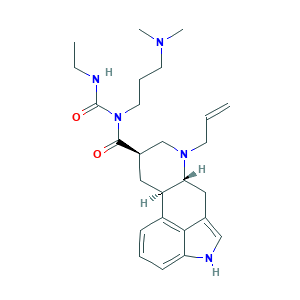
- C26H37N5O2
- Canonical SMILES
- CCNC(=O)N(CCCN(C)C)C(=O)C1CC2C(CC3=CNC4=CC=CC2=C34)N(C1)CC=C
- InChI
- 1S/C26H37N5O2/c1-5-11-30-17-19(25(32)31(26(33)27-6-2)13-8-12-29(3)4)14-21-20-9-7-10-22-24(20)18(16-28-22)15-23(21)30/h5,7,9-10,16,19,21,23,28H,1,6,8,11-15,17H2,2-4H3,(H,27,33)/t19-,21-,23-/m1/s1
- InChIKey
- KORNTPPJEAJQIU-KJXAQDMKSA-N
|