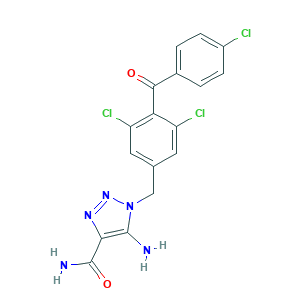
| Synonyms |
Carboxyamido-triazole; Carboxyamidotriazole; L 651582; L-651,582; L-651582; L651582; 4-CAI; 5-Amino-1-((3,5-dichloro-4-(4-chlorobenzoyl)phenyl)methyl)-1H-1,2,3-triazole-4-carboxamide; 5-amino-1-(3,5-dichloro-4-(4-chlorobenzoyl)benzyl)-1H-1,2,3-triazole-4-carboxamide; 5-amino-1-[[3,5-dichloro-4-(4-chlorobenzoyl)phenyl]methyl]triazole-4-carboxamide; 6ST3ZF52WB; 99519-84-3; C17H12Cl3N5O2; CAI; MLS003373924; NSC 609974; NSC-609974; NSC609974; SMR002048715; UNII-6ST3ZF52WB
|
| Cross-matching ID |
- PubChem CID
- 108144
- PubChem SID
-
485747
; 639419
; 4254373
; 6887112
; 8141938
; 10234614
; 10320678
; 11409172
; 11459118
; 11461236
; 12013671
; 14831891
; 26653376
; 44437032
; 50065444
; 50109733
; 57338464
; 58108226
; 80545111
; 92722332
; 103143867
; 103310486
; 104380609
; 117565421
; 123122707
; 124635539
; 124753387
; 124878929
; 125569773
; 126627359
; 126648997
; 126662661
; 129607850
; 134224803
; 135064836
; 135698191
; 137038061
; 140115329
; 143493287
; 152243201
; 162202203
; 162220752
; 162755062
; 164038435
; 164849207
; 171578519
; 174006266
; 196107484
; 198972622
; 198991783
- CAS Number
-
- TTD Drug ID
- D05IIP
- Formula
- C17H12Cl3N5O2
- Canonical SMILES
- C1=CC(=CC=C1C(=O)C2=C(C=C(C=C2Cl)CN3C(=C(N=N3)C(=O)N)N)Cl)Cl
- InChI
- 1S/C17H12Cl3N5O2/c18-10-3-1-9(2-4-10)15(26)13-11(19)5-8(6-12(13)20)7-25-16(21)14(17(22)27)23-24-25/h1-6H,7,21H2,(H2,22,27)
- InChIKey
- WNRZHQBJSXRYJK-UHFFFAOYSA-N
|