| Synonyms |
Corticosteron; Kendall's compound B; Reichstein's B; Reichstein's substance H; (11beta)-11,21-Dihydroxypregn-4-ene-3,20-dione; 11,12-Dihydroxyprogesterone; 11,21-Dihydroxyprogesterone; 11-Hydroxycorticoaldosterone; 11-beta,21-Dihydroxypregn-3,20-dione; 11Beta,21-dihydroxypregn-4-ene-3,20-dione; 11beta,21-Dihydroxy-4-pregnene-3,20-dione; CORTICOSTERONE; Compound B; 11beta,21-Dihydroxyprogesterone; 17-Deoxycortisol; 4-Pregnene-11beta,21-diol-3,20-dione; 50-22-6; CCRIS 6753; CHEBI:16827; NSC9705; UNII-W980KJ009P
|
| Cross-matching ID |
- PubChem CID
- 5753
- PubChem SID
-
5219
; 75084
; 804393
; 841767
; 855741
; 6435953
; 7886418
; 7978988
; 8143631
; 8153530
; 10321632
; 11466460
; 11467580
; 11486119
; 17389502
; 17404783
; 24278302
; 24856812
; 24869860
; 25630774
; 26751495
; 26758495
; 29224789
; 46386953
; 46504547
; 47275526
; 47646532
; 48018879
; 48243330
; 48318377
; 48393901
; 48393902
; 48421980
; 48424986
; 49698488
; 49956045
; 50104040
; 53777326
; 53788667
; 53834312
; 56311825
; 56312404
; 56312772
; 56313244
; 56423132
; 57322931
; 57390013
; 57648276
; 57650717
; 58685480
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03TNT
- Formula
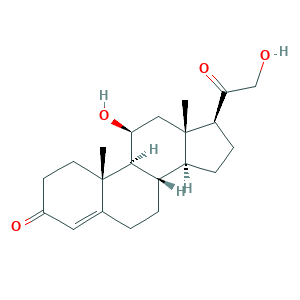
- C21H30O4
- Canonical SMILES
- CC12CCC(=O)C=C1CCC3C2C(CC4(C3CCC4C(=O)CO)C)O
- InChI
- 1S/C21H30O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h9,14-17,19,22,24H,3-8,10-11H2,1-2H3/t14-,15-,16+,17-,19+,20-,21-/m0/s1
- InChIKey
- OMFXVFTZEKFJBZ-HJTSIMOOSA-N
|