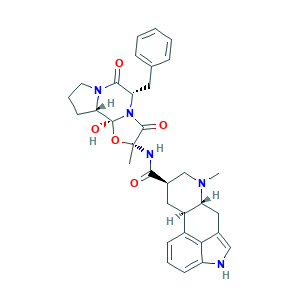
| Synonyms |
Dehydroergotamine; Dihidroergotamina; Dihidroergotamina [INN-Spanish]; Dihydergot; Dihydroergotamine mesylate; Dihydroergotamine methanesulfonate; Dihydroergotaminum; Dihydroergotaminum [INN-Latin]; Diidroergotamina; Diidroergotamina [DCIT]; Angionorm; DHE-45; Ergomimet; Ergotamine, 9,10-dihydro-; Levadex; Migranal; Orstanorm; Verladyn; dihydroergotamine; 436O5HM03C; 511-12-6; 9,10-Dihydroergotamine; 9,10-dihydro-ergotamine; BRN 5720196; C33H37N5O5; CHEBI:4562; CHEMBL1732; D.H.E. 45; EINECS 208-123-3; NCGC00017400-05; UNII-436O5HM03C
|
| Cross-matching ID |
- PubChem CID
- 10531
- PubChem SID
-
10000
; 7979087
; 8157604
; 11335767
; 11361006
; 11363951
; 11366513
; 11369075
; 11371529
; 11374714
; 11377237
; 11461978
; 11484971
; 11489004
; 11490211
; 11492773
; 11494871
; 14788984
; 14935810
; 29229015
; 46507711
; 47291112
; 47885391
; 48110437
; 48334472
; 49963030
; 49965612
; 50153282
; 57326281
; 85789540
; 90341683
; 93166278
; 96024534
; 99313633
; 103684622
; 103905875
; 104137088
; 104171355
; 104323551
; 119501400
; 124749650
; 124883197
; 124883198
; 126688923
; 127323995
; 127323996
; 127323997
; 127323998
; 127323999
; 127324000
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0V3ZA
- Formula
- C33H37N5O5
- Canonical SMILES
- CC1(C(=O)N2C(C(=O)N3CCCC3C2(O1)O)CC4=CC=CC=C4)NC(=O)C5CC6C(CC7=CNC8=CC=CC6=C78)N(C5)C
- InChI
- 1S/C33H37N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39)/t21-,23-,25-,26+,27+,32-,33+/m1/s1
- InChIKey
- LUZRJRNZXALNLM-JGRZULCMSA-N
|