| Synonyms |
Eliglustat (Tartrate); Eliglustat (hemitartrate); Eliglustat hemitartrate(Genz-99067); Eliglustat tartrate (JAN/USAN); Eliglustat tartrate [USAN]; Genz 112638; HY-14885A; N0493335P3; Octanamide, N-((1R,2R)-2-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-hydroxy-1-(1- pyrrolidinylmethyl)ethyl)-, (2R,3R)-2,3-dihydroxybutanedioate (2:1); SB16832; UNII-N0493335P3; Eliglustat L-tartrate; Eliglustat hemitartrate; 928659-70-5; CHEBI:83353; CS-5423; Cerdelga; Cerdelga (TN); DTXSID50239166; EX-A2301-1
|
| Cross-matching ID |
- PubChem CID
- 52918379
- PubChem SID
-
123055388
; 135264656
; 135626643
; 198956158
; 210024103
; 223447916
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J8IJ
- Formula
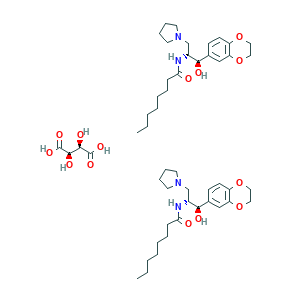
- C50H78N4O14
- Canonical SMILES
- CCCCCCCC(=O)NC(CN1CCCC1)C(C2=CC3=C(C=C2)OCCO3)O.CCCCCCCC(=O)NC(CN1CCCC1)C(C2=CC3=C(C=C2)OCCO3)O.C(C(C(=O)O)O)(C(=O)O)O
- InChI
- 1S/2C23H36N2O4.C4H6O6/c2*1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20;5-1(3(7)8)2(6)4(9)10/h2*10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26);1-2,5-6H,(H,7,8)(H,9,10)/t2*19-,23-;1-,2-/m111/s1
- InChIKey
- KUBARPMUNHKBIQ-VTHUDJRQSA-N
|