| Synonyms |
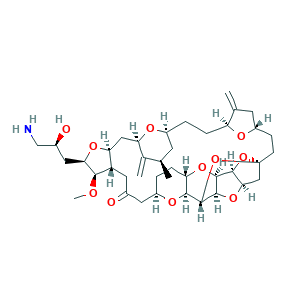
Eribulin; Eribulin [INN]; LR24G6354G; 2-(3-Amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9H,15H-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopentacosin-5-(4H)-one; ER 086526; 253128-41-5; CHEBI:63587; UNII-LR24G6354G
|
| Cross-matching ID |
- PubChem CID
- 11354606
- PubChem SID
-
16446284
; 135253408
; 135611179
; 164147247
; 175268313
; 178103419
; 210274856
; 210280489
; 240715658
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0KK2E
- Formula
- C40H59NO11
- Canonical SMILES
- CC1CC2CCC3C(=C)CC(O3)CCC45CC6C(O4)C7C(O6)C(O5)C8C(O7)CCC(O8)CC(=O)CC9C(CC(C1=C)O2)OC(C9OC)CC(CN)O
- InChI
- 1S/C40H59NO11/c1-19-11-24-5-7-28-20(2)12-26(45-28)9-10-40-17-33-36(51-40)37-38(50-33)39(52-40)35-29(49-37)8-6-25(47-35)13-22(42)14-27-31(16-30(46-24)21(19)3)48-32(34(27)44-4)15-23(43)18-41/h19,23-39,43H,2-3,5-18,41H2,1,4H3/t19-,23+,24+,25-,26+,27+,28+,29+,30-,31+,32-,33-,34-,35+,36+,37+,38-,39+,40+/m1/s1
- InChIKey
- UFNVPOGXISZXJD-JBQZKEIOSA-N
|