| Synonyms |
Etynodiol; Aethynodiolum; CHEBI:50785; CHEMBL1201406; DB13866; DTXSID1023025; EINECS 214-971-5; Ethinodiol; Etinodiol; Etinodiol [INN-Spanish]; Etinodiolo; Etinodiolo [DCIT]; Etynodiol (INN); Etynodiol [INN:BAN]; Etynodiolum; Etynodiolum [INN-Latin]; HSDB 7897; LS-97401; (3-beta,17-alpha)-19-Norpregn-4-en-20-yne-3,17-diol; (3beta,17alpha)-19-Norpregn-4-en-20-yne-3,17-diol; (3beta,17beta)-17-ethynylestr-4-ene-3,17-diol; 1231-93-2; 17-alpha-Ethynyl-19-norandrost-4-ene-3-beta,17-beta-diol; 17alpha-Ethynyl-19-norandrost-4-ene-3beta,17beta-diol; 17alpha-Ethynyl-estra-4-ene-3beta,17beta-diol; 17alpha-ethynylestr-4-ene-3beta,17beta-diol; 19-Nor-17-alpha-pregn-4-en-20-yne-3-beta,17-diol; 3beta-hydroxynorethisterone; 9E01C36A9S; SCHEMBL140933; UNII-9E01C36A9S; ZINC30691629; ethynodiol; ZINC000030691629
|
| Cross-matching ID |
- PubChem CID
- 14309340
- CAS Number
-
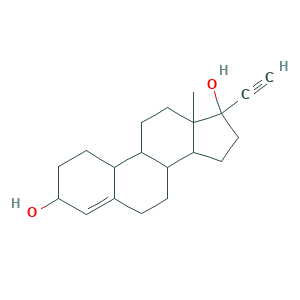
- Formula
- C20H28O2
- Canonical SMILES
- CC12CCC3C(C1CCC2(C#C)O)CCC4=CC(CCC34)O
- InChI
- 1S/C20H28O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,12,14-18,21-22H,4-11H2,2H3
- InChIKey
- JYILPERKVHXLNF-UHFFFAOYSA-N
|