| Synonyms |
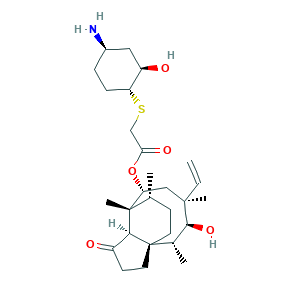
Lefamulin; Lefamulin [INN]; Lefamulin(BC-3781); 1061337-51-6; Acetic acid, 2-(((1R,2R,4R)-4-amino-2-hydroxycyclohexyl)thio)-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3ah-cyclopentacycloocten-8-yl ester; Acetic acid, 2-[[(1R,2R,4R)-4-amino-2-hydroxycyclohexyl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester; CHEMBL3291398; UNII-21904A5386
|
| Cross-matching ID |
- PubChem CID
- 25185057
- CAS Number
-
- TTD Drug ID
- D0EB5N
- Formula
- C28H45NO5S
- Canonical SMILES
- CC1CCC23CCC(=O)C2C1(C(CC(C(C3C)O)(C)C=C)OC(=O)CSC4CCC(CC4O)N)C
- InChI
- 1S/C28H45NO5S/c1-6-26(4)14-22(34-23(32)15-35-21-8-7-18(29)13-20(21)31)27(5)16(2)9-11-28(17(3)25(26)33)12-10-19(30)24(27)28/h6,16-18,20-22,24-25,31,33H,1,7-15,29H2,2-5H3/t16-,17+,18-,20-,21-,22-,24+,25+,26-,27+,28+/m1/s1
- InChIKey
- KPVIXBKIJXZQJX-FCEONZPQSA-N
|