| General Information of Drug (ID:
DR0965) |
| Drug Name |
Lisuride
|
| Synonyms |
LISURIDE; Lisurida; Lisurida [INN-Spanish]; Lisuride (INN); Lisuride (S)(-); Lisuride [INN]; Lisuridum; Lisuridum [INN-Latin]; Lysurid; Lysuride; Methylergol Carbamide; lisuride maleate; 1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea; 18016-80-3; 3-(9,10-Didehydro-6-methylergolin-8alpha-yl)-1,1-diethylurea; C20H26N4O; CHEBI:51164; CHEMBL157138; E0QN3D755O; EINECS 241-925-1; N'-((8alpha)-9,10-Didehydro-6-methylergolin-8-yl)-N,N-diethylurea; UNII-E0QN3D755O
|
| Indication |
Parkinsonism
[ICD11: 8A00]
|
Phase 3
|
[1]
|
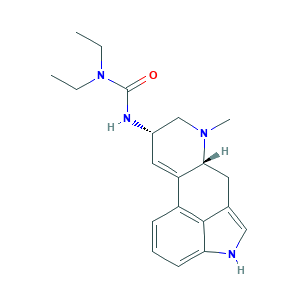
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
338.4 |
Topological Polar Surface Area |
51.4 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 28864
- PubChem SID
-
7979782
; 8170644
; 10321508
; 11466134
; 11467254
; 11485767
; 14827074
; 16304643
; 34671274
; 46505557
; 47216935
; 47440423
; 47515485
; 47810926
; 47885573
; 48416176
; 49698348
; 50060231
; 56464344
; 57309764
; 57310710
; 76836308
; 92125058
; 96024822
; 103408740
; 103914001
; 103952701
; 104303892
; 124749968
; 124893624
; 127339697
; 127339698
; 127339699
; 127339700
; 127339701
; 127529620
; 134337569
; 134340181
; 134340461
; 135269077
; 135650525
; 137001465
; 137225007
; 142950042
; 160963934
; 163304387
; 164761611
; 179150133
; 224890570
; 226428258
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X7KB
- Formula
- C20H26N4O
- Canonical SMILES
- CCN(CC)C(=O)NC1CN(C2CC3=CNC4=CC=CC(=C34)C2=C1)C
- InChI
- 1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18+/m0/s1
- InChIKey
- BKRGVLQUQGGVSM-KBXCAEBGSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


