| Cross-matching ID |
- PubChem CID
- 247839
- PubChem SID
-
109976
; 7849348
; 7979880
; 9395740
; 10321190
; 11466771
; 11467891
; 11486401
; 11533031
; 14827409
; 17395643
; 24896551
; 30046333
; 46506584
; 47276848
; 47425535
; 47500776
; 47647746
; 48020242
; 48170674
; 49698627
; 50124175
; 50322454
; 56423131
; 57400476
; 71825554
; 85148347
; 85788382
; 89850274
; 92125692
; 95613570
; 103771156
; 103913670
; 104170097
; 104493518
; 121362376
; 121363363
; 124583836
; 124800109
; 128027553
; 134338310
; 134983239
; 137001649
; 144204077
; 160963601
; 170465194
; 172080722
; 175266363
; 175611984
; 178103664
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04SFH
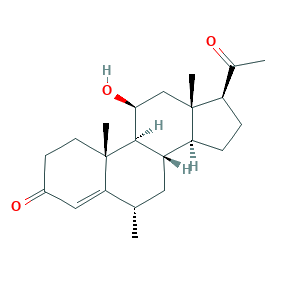
- Formula
- C22H32O3
- Canonical SMILES
- CC1CC2C3CCC(C3(CC(C2C4(C1=CC(=O)CC4)C)O)C)C(=O)C
- InChI
- 1S/C22H32O3/c1-12-9-15-17-6-5-16(13(2)23)22(17,4)11-19(25)20(15)21(3)8-7-14(24)10-18(12)21/h10,12,15-17,19-20,25H,5-9,11H2,1-4H3/t12-,15-,16+,17-,19-,20+,21-,22+/m0/s1
- InChIKey
- GZENKSODFLBBHQ-ILSZZQPISA-N
|