| Cross-matching ID |
- PubChem CID
- 8226
- PubChem SID
-
7979943
; 8155761
; 11335532
; 11360771
; 11363157
; 11365719
; 11368281
; 11371623
; 11376443
; 11461743
; 11484282
; 11488429
; 11490318
; 11494077
; 14777917
; 26751766
; 29226945
; 46507746
; 47216702
; 47515240
; 47588916
; 47662201
; 47885332
; 47885333
; 48110372
; 48184922
; 48334408
; 48416242
; 49699341
; 49965412
; 50104453
; 57324864
; 75350935
; 85788317
; 85789357
; 90341291
; 92729787
; 96024895
; 104171343
; 104317266
; 123085521
; 123109975
; 124750004
; 124882912
; 124882913
; 127346532
; 127346533
; 134338249
; 134973870
; 135650591
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05AHE
- Formula
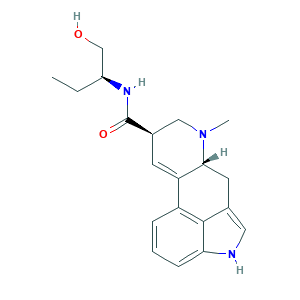
- C20H25N3O2
- Canonical SMILES
- CCC(CO)NC(=O)C1CN(C2CC3=CNC4=CC=CC(=C34)C2=C1)C
- InChI
- 1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1
- InChIKey
- UNBRKDKAWYKMIV-QWQRMKEZSA-N
|