| Synonyms |
Macroral; Midecamycin acetate (JP17); Midecamycin acetate [JAN]; Miocamen; Miocamycin; Miocamycin (TN); Miocamycine; Miokamycin; Myocamicin; Ponsinomycin; SCHEMBL139293; ZINC169677000; midecamycin acetate; 3'',9-Diacetylmydecamycin; 3T48CPS7U2; 55881-07-7; 9,3''-Di-O-Acetylmidecamycin; 9,3''-Diacetylmidecamycin; AC1NQZMS; Acecamycin; C45H71NO17; CHEMBL1091024; DTXSID60905087; EINECS 259-879-6; Leucomycin V, 3(sup B),9-diacetate 3,4(sup B)-dipropanoate; Leucomycin V, 3B,9-diacetate 3,4B-dipropanoate; MOM; Mosil; UNII-3T48CPS7U2
|
| Cross-matching ID |
- PubChem CID
- 5282188
- CAS Number
-
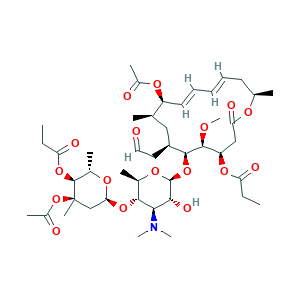
- Formula
- C45H71NO17
- Canonical SMILES
- CCC(=O)OC1CC(=O)OC(CC=CC=CC(C(CC(C(C1OC)OC2C(C(C(C(O2)C)OC3CC(C(C(O3)C)OC(=O)CC)(C)OC(=O)C)N(C)C)O)CC=O)C)OC(=O)C)C
- InChI
- 1S/C45H71NO17/c1-13-34(50)59-33-23-36(52)55-26(4)18-16-15-17-19-32(58-29(7)48)25(3)22-31(20-21-47)41(42(33)54-12)62-44-39(53)38(46(10)11)40(27(5)57-44)61-37-24-45(9,63-30(8)49)43(28(6)56-37)60-35(51)14-2/h15-17,19,21,25-28,31-33,37-44,53H,13-14,18,20,22-24H2,1-12H3/b16-15+,19-17+/t25-,26-,27-,28+,31+,32+,33-,37+,38-,39-,40-,41+,42+,43+,44+,45-/m1/s1
- InChIKey
- GQNZGCARKRHPOH-RQIKCTSVSA-N
|