| Synonyms |
Rufinamide; WFW942PR79; 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide; 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-Triazole-4-carboxamide; Banzel; Inovelon; POGQSBRIGCQNEG-UHFFFAOYSA-N; RUF 331; RUF-331; 1-[(2,6-difluorophenyl)methyl]triazole-4-carboxamide; 106308-44-5; 1H-1,2,3-Triazole-4-carboxamide, 1-[(2,6-difluorophenyl)methyl]-; C10H8F2N4O; CGP-33101; Cgp 33101; DSSTox_CID_26506; DSSTox_GSID_46506; DSSTox_RID_81675; E 2080; E2080; MFCD00865314; NCGC00165883-02; UNII-WFW942PR79; Xilep
|
| Cross-matching ID |
- PubChem CID
- 129228
- PubChem SID
-
10242268
; 12014051
; 15121846
; 24724595
; 29217641
; 29308110
; 47207436
; 50111716
; 56320687
; 56323499
; 57342925
; 58107211
; 80334982
; 90341717
; 90342554
; 92718980
; 93309661
; 99437055
; 103771489
; 104369993
; 109693097
; 118317931
; 121361738
; 124757121
; 124772079
; 124800269
; 125163925
; 125337281
; 126600262
; 126621181
; 126651848
; 126667055
; 128944616
; 131293789
; 134338700
; 134340299
; 135085479
; 135653558
; 135698201
; 136343339
; 137027833
; 137255007
; 142190226
; 143493273
; 144205820
; 152241718
; 162037521
; 162184408
; 163124160
; 163564750
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0MD2L
- Formula
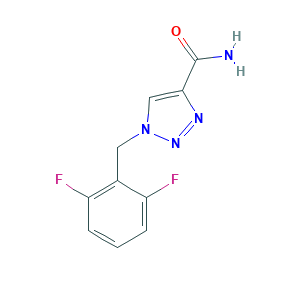
- C10H8F2N4O
- Canonical SMILES
- C1=CC(=C(C(=C1)F)CN2C=C(N=N2)C(=O)N)F
- InChI
- 1S/C10H8F2N4O/c11-7-2-1-3-8(12)6(7)4-16-5-9(10(13)17)14-15-16/h1-3,5H,4H2,(H2,13,17)
- InChIKey
- POGQSBRIGCQNEG-UHFFFAOYSA-N
|