| General Information of Drug (ID:
DR1508) |
| Drug Name |
Sufentanil
|
| Synonyms |
Sufentanilum; Sufentanilum [INN-Latin]; Sufentanyl; Sulfentanil; Sulfentanyl; Zalviso; 56030-54-7; C22H30N2O2S; CHEBI:9316; AFE2YW0IIZ; Chronogesic; GGCSSNBKKAUURC-UHFFFAOYSA-N; R 30730; R-30730; SUFENTANIL; HSDB 6760; N-(4-(Methoxymethyl)-1-(2-(2-thienyl)ethyl)-4-piperidinyl)-N-phenylpropanamide; N-(4-(Methoxymethyl)-1-(2-(2-thienyl)ethyl)-4-piperidyl)propionanilide; N-[4-(methoxymethyl)-1-(2-thiophen-2-ylethyl)piperidin-4-yl]-N-phenylpropanamide; UNII-AFE2YW0IIZ
|
| Indication |
Neuropathic pain
[ICD11: 8E43]
|
Approved
|
[1]
|
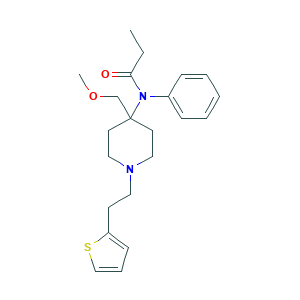
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
386.6 |
Topological Polar Surface Area |
61 |
| Heavy Atom Count |
27 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 41693
- PubChem SID
-
10222
; 6311194
; 7980695
; 8177237
; 14804981
; 34707309
; 46504737
; 47207596
; 47252825
; 47326870
; 47624342
; 47846995
; 48416563
; 50064909
; 50787758
; 57312595
; 85209320
; 103189274
; 103924757
; 104337672
; 111978170
; 117568208
; 124953716
; 124953717
; 127949007
; 134222396
; 134337667
; 135003073
; 137002461
; 139212423
; 160964052
; 164035747
; 175268924
; 175443463
; 178100513
; 179150640
; 185971931
; 198991634
; 210274958
; 210280596
; 223398861
; 224247843
; 226414514
; 241097876
; 250113166
; 251880715
; 252481124
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D8DD
- Formula
- C22H30N2O2S
- Canonical SMILES
- CCC(=O)N(C1=CC=CC=C1)C2(CCN(CC2)CCC3=CC=CS3)COC
- InChI
- 1S/C22H30N2O2S/c1-3-21(25)24(19-8-5-4-6-9-19)22(18-26-2)12-15-23(16-13-22)14-11-20-10-7-17-27-20/h4-10,17H,3,11-16,18H2,1-2H3
- InChIKey
- GGCSSNBKKAUURC-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


