| Synonyms |
Tafenoquine; Tafenoquine [INN:BAN]; Arakoda; Etaquine; Krintafel; SB-252263; WR 238605; WR-238605; WR238605; (R)-N3-(2,6-Dimethoxy-4-methyl-5-(3-trifluoromethyl)phenoxy)quinolin-8-yl)pentane-1,4-diamine; (RS)-N(sup 3)-(2,6-Dimethoxy-4-methyl-5-(3-trifluoro-methylphenoxy)quinolin-8-yl)pentane-1,4-diamine; 1,4-Pentanediamine, N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)-8-quinolinyl)-; 106635-80-7; N(4)-(2,6-Dimethoxy-4-methyl-5-((3-trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine
|
| Cross-matching ID |
- PubChem CID
- 115358
- PubChem SID
-
602533
; 10236751
; 14784788
; 29296849
; 50064119
; 50683558
; 57339134
; 103230320
; 104398192
; 112378993
; 128464465
; 135072665
; 137249474
; 139445279
; 172232583
; 179148060
; 224112192
; 226682048
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07TWN
- Formula
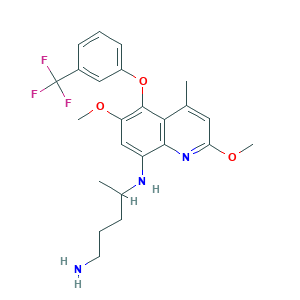
- C24H28F3N3O3
- Canonical SMILES
- CC1=CC(=NC2=C1C(=C(C=C2NC(C)CCCN)OC)OC3=CC=CC(=C3)C(F)(F)F)OC
- InChI
- 1S/C24H28F3N3O3/c1-14-11-20(32-4)30-22-18(29-15(2)7-6-10-28)13-19(31-3)23(21(14)22)33-17-9-5-8-16(12-17)24(25,26)27/h5,8-9,11-13,15,29H,6-7,10,28H2,1-4H3
- InChIKey
- LBHLFPGPEGDCJG-UHFFFAOYSA-N
|