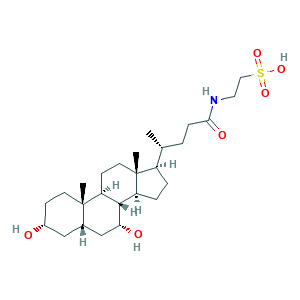
| Synonyms |
Chenodeoxycholoyltaurine; Chenodeoxycholyltaurine; Taurochenodeoxycholic acid; TAUROCHENODEOXYCHOLIC ACID; Taurine chenodeoxycholate; Taurochenodeoxycholate; Taurochenodesoxycholate; Taurochenodesoxycholic acid; 12-Deoxycholyltaurine; 12-Desoxycholyltaurine; 516-35-8; 651KU15938; C26H45NO6S; CHEBI:16525; CHEMBL185878; Ethanesulfonic acid, 2-(((3alpha,5beta,7alpha)-3,7-dihydroxy-24-oxocholan-24-yl)amino)-; N-(3alpha,7alpha-dihydroxy-5beta-cholan-24-oyl)-taurine; TCDCA; TUD; UNII-651KU15938; n-(3a,7a-dihydroxy-5b-cholan-24-oyl)-taurine
|
| Cross-matching ID |
- PubChem CID
- 387316
- PubChem SID
-
7825
; 8143524
; 11433252
; 11566423
; 15680651
; 17422072
; 17422074
; 24702369
; 50246905
; 53789838
; 57402301
; 103457854
; 103832585
; 104602663
; 119525713
; 126523702
; 134976110
; 136073242
; 137105399
; 138870429
; 160854685
; 162224001
; 178101457
; 179293218
; 196383650
; 226439344
; 241089240
; 249868382
; 252067842
; 252500397
; 252811091
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02YJL
- Formula
- C26H45NO6S
- Canonical SMILES
- CC(CCC(=O)NCCS(=O)(=O)O)C1CCC2C1(CCC3C2C(CC4C3(CCC(C4)O)C)O)C
- InChI
- 1S/C26H45NO6S/c1-16(4-7-23(30)27-12-13-34(31,32)33)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(28)14-17(25)15-22(24)29/h16-22,24,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17+,18-,19-,20+,21+,22-,24+,25+,26-/m1/s1
- InChIKey
- BHTRKEVKTKCXOH-BJLOMENOSA-N
|