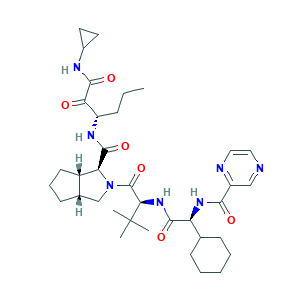
| Synonyms |
Telaprevir; Telaprevir (VX-950); Telavic; VRT-111950; VX 950; VX-950; VX-950(Telaprevir); Incivek; Incivo; LY-570310; MP-424; S-Telaprevir; (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-6-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide; 402957-28-2; 655M5O3W0U; CHEBI:68595; CHEMBL231813; UNII-655M5O3W0U
|
| Cross-matching ID |
- PubChem CID
- 3010818
- PubChem SID
-
3723201
; 10039409
; 14839657
; 16347884
; 36059235
; 49684228
; 80491883
; 96025695
; 99455178
; 103533130
; 109693015
; 111630651
; 123105171
; 126578521
; 126666050
; 126727848
; 134223970
; 134340525
; 134340605
; 135692887
; 136920427
; 137188810
; 137273793
; 141479428
; 143498830
; 152234951
; 152258142
; 152344432
; 160645797
; 160646981
; 162011420
; 162197409
; 163884617
; 164193943
; 164765234
; 175266405
; 175426470
; 175427024
; 176250091
; 184812119
; 198992420
; 223366200
; 223385689
; 223669631
; 224452297
; 226544280
; 248618622
; 249810645
; 249821696
; 251916476
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X9CH
- Formula
- C36H53N7O6
- Canonical SMILES
- CCCC(C(=O)C(=O)NC1CC1)NC(=O)C2C3CCCC3CN2C(=O)C(C(C)(C)C)NC(=O)C(C4CCCCC4)NC(=O)C5=NC=CN=C5
- InChI
- 1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25-,27-,28-,30+/m0/s1
- InChIKey
- BBAWEDCPNXPBQM-GDEBMMAJSA-N
|