| Synonyms |
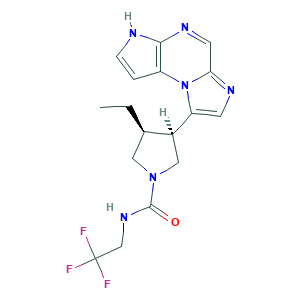
Upadacitinib, ABT-494; J3.590.729G; Rinvoq; SB19218; SCHEMBL9991056; Upadacitinib; Upadacitinib (USAN/INN); Upadacitinib [USAN:INN]; 1-Pyrrolidinecarboxamide, 3-ethyl-4-(3H-imidazo(1,2-a)pyrrolo(2,3-e)pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-, (3S,4R)-; 1310726-59-0; 1310726-60-3; 4RA0KN46E0; ABT 494; ABT-494; ABT-494 enantiomer; AC-30326; BCP19011; CHEMBL3622821; CS-6150; D10994; EX-A1628; GTPL9246; HY-19569; MFCD30502663; UNII-4RA0KN46E0
|
| Cross-matching ID |
- PubChem CID
- 58557659
- CAS Number
-
- Formula
- C17H19F3N6O
- Canonical SMILES
- CCC1CN(CC1C2=CN=C3N2C4=C(NC=C4)N=C3)C(=O)NCC(F)(F)F
- InChI
- 1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1
- InChIKey
- WYQFJHHDOKWSHR-MNOVXSKESA-N
|