| Synonyms |
Vinorelbine Bitartrate; Vinorelbine ditartrate; Vinorelbine tartrate; Vinorelbine L-tartrate; Vinorelbine ditartaric acid; Vinorelbine, ditartrate; Vinorelbina; Vinorelbina [Spanish]; Vinorelbine (INN); Vinorelbine [INN:BAN]; Vinorelbine(Navelbine); Vinorelbinum; Vinorelbinum [Latin]; methyl (2b,3b,4b,5a,12b,19a)-4-(acetyloxy)-15-[(6R,8S)-4-ethyl-8-(methoxycarbonyl)-1,3,6,7,8,9-hexahydro-2,6-methanoazecino[4,3-b]indol-8-yl]-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3-carboxylate; Vinorelbine; 71486-22-1; C45H54N4O8; CHEBI:480999; Eunades; Exelbine; KW 2307 base; KW-2307; Navelbine; Navelbine (TN); Nor-5'-anhydrovinblastine; Q6C979R91Y; UNII-Q6C979R91Y; 125317-39-7; 3',4'-DIDEHYDRO-4'-DEOXY-C'-NORVINCALEUKOBLASTINE R-(R*,R*)-2-3-DIHYDROXYBUTANEDIOATE (1:2) SALT; 3',4'-Didehydro-4'-deoxy-C'-norvincaleukoblastine [R-(R*,R*)-2-3-dihydroxybutanedioate (1:2)salt]; 317V397; 5'-Noranhydrovinoblastine tartrate; AB15572; MFCD03613607; NVB; Navelbine tartrate; Q-100110; VINORELBINE DITARTRATE SALT
|
| Cross-matching ID |
- PubChem CID
- 45055483
- CAS Number
-
- TTD Drug ID
- D01HTL
- Formula
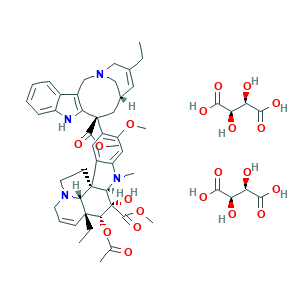
- C53H66N4O20
- Canonical SMILES
- CCC1=CC2CC(C3=C(CN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC.C(C(C(=O)O)O)(C(=O)O)O.C(C(C(=O)O)O)(C(=O)O)O
- InChI
- 1S/C45H54N4O8.2C4H6O6/c1-8-27-19-28-22-44(40(51)55-6,36-30(25-48(23-27)24-28)29-13-10-11-14-33(29)46-36)32-20-31-34(21-35(32)54-5)47(4)38-43(31)16-18-49-17-12-15-42(9-2,37(43)49)39(57-26(3)50)45(38,53)41(52)56-7;2*5-1(3(7)8)2(6)4(9)10/h10-15,19-21,28,37-39,46,53H,8-9,16-18,22-25H2,1-7H3;2*1-2,5-6H,(H,7,8)(H,9,10)/t28-,37+,38-,39-,42-,43-,44+,45+;2*1-,2-/m111/s1
- InChIKey
- CILBMBUYJCWATM-LNLXBTRNSA-N
|