| Synonyms |
Floxuridine; 5-Fluorodeoxyuridine; 2'-Deoxy-5-fluorouridine; Fluorodeoxyuridine; FUDR; Floxuridin; Deoxyfluorouridine; Fluoruridine deoxyribose; 5-Fluoro-2'-deoxyuridine; 5FdU; Floxuridinum; Floxiridina; 5-Fluoro-2-desoxyuridine; FdUrd; 5 Fluorodeoxyuridine; beta-5-Fluoro-2'-deoxyuridine; Floxuridinum [INN-Latin]; 5-Fluorouracil 2'-deoxyriboside; Floxiridina [INN-Spanish]; 5-fluoro-1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1h,3h)-dione; 5-Fluoro-2-deoxyuridine; 5-Fluorouracil deoxyriboside; 5-FdUrd; 1-(2-Deoxy-beta-D-ribofuranosyl)-5-fluorouracil; 1beta-D-2'-Deoxyribofuranosyl-5-flurouracil; 1-beta-D-2'-Deoxyribofuranosyl-5-flurouracil; Uridine, 2'-deoxy-5-fluoro-; NSC-27640; FDUR; UNII-039LU44I5M; 5-fluoro-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,3,4-tetrahydropyrimidine-2,4-dione; (+)-5-fluoro-2'-deoxyuridine; CHEBI:60761; HSDB 3227; 5-FUDR; EINECS 200-072-5; (+)-5-fluorodeoxyuridine; CHEMBL917; BRN 0090221; AI3-50691; MLS000069439; 039LU44I5M; NSC 27640; Uridine, 2'-deoxy-5'-fluoro-; NCGC00023722-05; SMR000059051; DSSTox_CID_3057; 5-Fluor-1-(beta-2'-deoxyribofuranosyl)pyrimidin-2,4(1H,3H)-dion [Czech]; Ro 5-0360; DSSTox_RID_76855; DSSTox_GSID_23057; FdU; Uridine, 2'-deoxy-5-fluoro; (+)-5-Fluoro-2 -deoxyuridine; CAS-50-91-9; Floxuridine [USAN:INN]; FUDR (TN); 5-Fluoro-2'-deoxy-beta-uridine; floxidine; Fluoxuridine; NSC27640; Floxuridine [USAN:USP:INN]; 5-floxuridine; Floxuridine,(S); 5-Fluoro-dUrd; MFCD00006530; Floxuridine (Fludara); Floxuridine (USP/INN); 50-91-9
|
| Cross-matching ID |
- PubChem CID
- 5790
- PubChem SID
-
13901
; 603423
; 855946
; 3139662
; 7978599
; 8153561
; 12146088
; 14749704
; 14749705
; 24894724
; 25622076
; 29215018
; 29215019
; 29224824
; 46508645
; 47206143
; 48416016
; 49693295
; 49833282
; 50061739
; 50104188
; 50895505
; 56459391
; 57322956
; 85279351
; 87568673
; 92309168
; 92714934
; 93576919
; 103158493
; 103240093
; 104310265
; 117613653
; 124558526
; 124799590
; 126623741
; 126943727
; 127301295
; 127301296
; 127301297
; 127301298
; 127301299
; 127301300
; 127301301
; 127301302
; 127301303
; 127301304
; 127301305
; 127301306
; 127301307
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0TS1Z
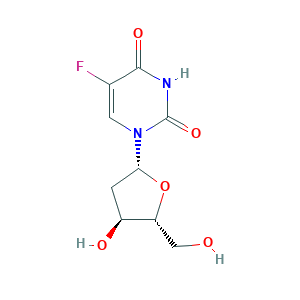
- Formula
- C9H11FN2O5
- Canonical SMILES
- C1C(C(OC1N2C=C(C(=O)NC2=O)F)CO)O
- InChI
- 1S/C9H11FN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
- InChIKey
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N
|