| Cross-matching ID |
- PubChem CID
- 6446
- PubChem SID
-
75841
; 76923
; 3133709
; 7847393
; 7979262
; 8154109
; 12159208
; 15988956
; 17388998
; 24702277
; 24894947
; 29225425
; 46508867
; 46518295
; 48425150
; 49869459
; 50458251
; 56394864
; 56422069
; 57323449
; 103516450
; 104312087
; 124813320
; 124890361
; 126678043
; 127336739
; 127336740
; 127336741
; 127336742
; 127336743
; 129813610
; 131325851
; 134222291
; 134338181
; 134971676
; 135650271
; 136903793
; 137003909
; 139326883
; 144206709
; 144208052
; 152034528
; 152164564
; 152250278
; 160964519
; 164788458
; 175265297
; 179296051
; 223680831
; 226396927
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L2LS
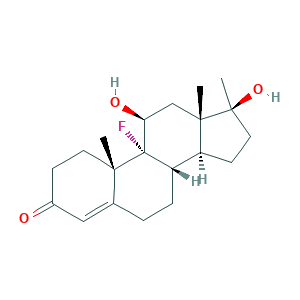
- Formula
- C20H29FO3
- Canonical SMILES
- CC12CCC(=O)C=C1CCC3C2(C(CC4(C3CCC4(C)O)C)O)F
- InChI
- 1S/C20H29FO3/c1-17-8-6-13(22)10-12(17)4-5-15-14-7-9-19(3,24)18(14,2)11-16(23)20(15,17)21/h10,14-16,23-24H,4-9,11H2,1-3H3/t14-,15-,16-,17-,18-,19-,20-/m0/s1
- InChIKey
- YLRFCQOZQXIBAB-RBZZARIASA-N
|