| General Information of Drug (ID:
DR1795) |
| Drug Name |
EPO-906
|
| Synonyms |
Epothilon B; Epothilone B; Epothilone B (EPO906, Patupilone); Epothilone-B; GNF-PF-193; Patupilone; Patupilone (EPO906, Epothilone B); Patupilone [INN]; UEC0H0URSE; (-)-Epothilone B; 152044-54-7; 7,11-DIHYDROXY-8,8,10,12,16-PENTAMETHYL-3-[1-METHYL-2-(2-METHYL-THIAZOL-4-YL)VINYL]-4,17-DIOXABICYCLO[14.1.0]HEPTADECANE-5,9-DIONE; CHEBI:31550; Epo B; EpoB; MFCD02101921; EPO 906; EPO 906A; EPO906; UNII-UEC0H0URSE
|
| Indication |
Breast cancer
[ICD11: 2C60]
|
Discontinued
|
[1]
|
| Structure |
|
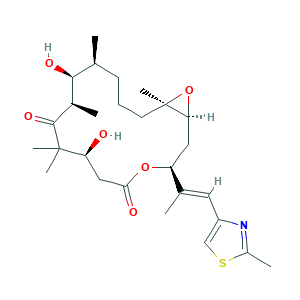 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
507.7 |
Topological Polar Surface Area |
138 |
| Heavy Atom Count |
35 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 448013
- PubChem SID
-
14301
; 520207
; 830084
; 7887332
; 10300156
; 12014962
; 14762393
; 14860221
; 17424973
; 24724475
; 36553605
; 49963244
; 50125842
; 57404848
; 74380521
; 80868833
; 99437114
; 103306054
; 104638848
; 124772091
; 125163996
; 126623079
; 126671632
; 131299984
; 131333506
; 131480855
; 136367402
; 136367813
; 137262638
; 142438244
; 152090491
; 152258740
; 152344296
; 160647585
; 162011973
; 162037563
; 162172155
; 164841003
; 172125880
; 177748943
; 179117109
; 184812277
; 198964552
; 221678840
; 223404331
; 223660093
; 223702472
; 226395914
; 243996700
; 249719024
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0CV7Z
- Formula
- C27H41NO6S
- Canonical SMILES
- CC1CCCC2(C(O2)CC(OC(=O)CC(C(C(=O)C(C1O)C)(C)C)O)C(=CC3=CSC(=N3)C)C)C
- InChI
- 1S/C27H41NO6S/c1-15-9-8-10-27(7)22(34-27)12-20(16(2)11-19-14-35-18(4)28-19)33-23(30)13-21(29)26(5,6)25(32)17(3)24(15)31/h11,14-15,17,20-22,24,29,31H,8-10,12-13H2,1-7H3/b16-11+/t15-,17+,20-,21-,22-,24-,27+/m0/s1
- InChIKey
- QXRSDHAAWVKZLJ-PVYNADRNSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


