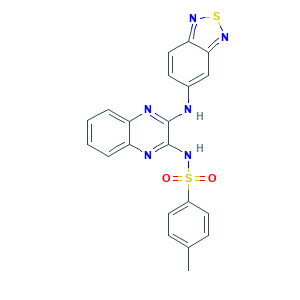
| Synonyms |
PI3K inhibitor X; Pilaralisib analogue; PubChem22457; XL 147; XL-147 derivative 2; XL147 analogue; 1033110-57-4; 956958-53-5; AC1LZ6F0; AC1Q2LO8; Benzenesulfonamide, N-[3-(2,1,3-benzothiadiazol-5-ylamino)-2-quinoxalinyl]-4-methyl-; C21H16N6O2S2; CHEBI:71957; N-(3-(benzo[c][1,2,5]thiadiazol-5-ylamino)quinoxalin-2-yl)-4-methylbenzenesulfonamide; N-[3-(2,1,3-Benzothiadiazol-5-ylamino)-2-quinoxalinyl]-4-methylbenzenesulfonamide; N-[3-(2,1,3-benzothiadiazol-5-ylamino)quinoxalin-2-yl]-4-methylbenzenesulfonamide; XL147; cc-43
|
| Cross-matching ID |
- PubChem CID
- 1893730
- PubChem SID
-
2292548
; 7613915
; 9162512
; 25687318
; 32753982
; 47324138
; 53012507
; 85758375
; 88887486
; 99436990
; 110757007
; 117413884
; 124757013
; 125163817
; 125655166
; 126626058
; 126658546
; 126729189
; 131480692
; 134964436
; 135698922
; 136340109
; 136367315
; 137102005
; 140639648
; 143499694
; 151981971
; 152234899
; 152258322
; 152344014
; 160647161
; 160962901
; 162011393
; 162037447
; 162202599
; 163772970
; 163907945
; 164042763
; 164077913
; 164825243
; 164836213
; 166698477
; 170503349
; 174006505
; 174531402
; 177748775
; 185967759
; 215730822
; 223388734
; 223686141
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03KAC
- Formula
- C21H16N6O2S2
- Canonical SMILES
- CC1=CC=C(C=C1)S(=O)(=O)NC2=NC3=CC=CC=C3N=C2NC4=CC5=NSN=C5C=C4
- InChI
- 1S/C21H16N6O2S2/c1-13-6-9-15(10-7-13)31(28,29)27-21-20(23-16-4-2-3-5-17(16)24-21)22-14-8-11-18-19(12-14)26-30-25-18/h2-12H,1H3,(H,22,23)(H,24,27)
- InChIKey
- MQMKRQLTIWPEDM-UHFFFAOYSA-N
|