| Synonyms |
NOV-002; Bi(glutathion-S-yl); Glutathiol; Glutathione disulphide; Glutathione, oxidized; Glutathione-S-S-glutathione; Glutathione-ssg; Glutathone disulfide; L(-)-Glutathione; L-Glutathione oxidized; L-Oxidized glutathione; OXIDIZED GLUTATHIONE DISULFIDE; Oxidized L-glutathione; Oxiglutationa [INN-Spanish]; Oxiglutatione [INN]; Oxiglutationum [INN-Latin]; Oxigluthione; S,S'-Ethylenebis(glutathione); glutathione disulfide; glutathione oxidized; oxidized glutathione; oxiglutatione; 27025-41-8; CCRIS 780; GSSG; UNII-ULW86O013H
|
| Cross-matching ID |
- PubChem CID
- 65359
- PubChem SID
-
3427
; 585870
; 806879
; 841098
; 3136528
; 7847099
; 7887808
; 7887987
; 8145606
; 8189646
; 14789556
; 14838688
; 24849044
; 24873116
; 24873118
; 24895168
; 24895174
; 24895265
; 26715683
; 26754399
; 29215201
; 43122165
; 48493821
; 49973708
; 53789868
; 57315456
; 57651219
; 75931676
; 81044516
; 81065831
; 85164993
; 87325785
; 91689687
; 92309454
; 96100227
; 99452992
; 103458646
; 104333553
; 118048837
; 124570891
; 126596846
; 129831439
; 134222313
; 134340058
; 134340320
; 135022908
; 135074887
; 137202462
; 137262620
; 141537993
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07BDD
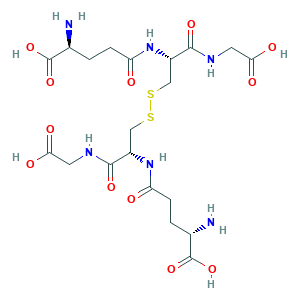
- Formula
- C20H32N6O12S2
- Canonical SMILES
- C(CC(=O)NC(CSSCC(C(=O)NCC(=O)O)NC(=O)CCC(C(=O)O)N)C(=O)NCC(=O)O)C(C(=O)O)N
- InChI
- 1S/C20H32N6O12S2/c21-9(19(35)36)1-3-13(27)25-11(17(33)23-5-15(29)30)7-39-40-8-12(18(34)24-6-16(31)32)26-14(28)4-2-10(22)20(37)38/h9-12H,1-8,21-22H2,(H,23,33)(H,24,34)(H,25,27)(H,26,28)(H,29,30)(H,31,32)(H,35,36)(H,37,38)/t9-,10-,11-,12-/m0/s1
- InChIKey
- YPZRWBKMTBYPTK-BJDJZHNGSA-N
|