| General Information of Drug (ID:
DR2123) |
| Drug Name |
Phosphoenolpyruvate
|
| Synonyms |
P-enol-pyruvate; P-enolpyruvate; Phospho(enol)pyruvic acid; Phosphoenolpyruvic acid; Phosphopyruvic acid; phosphoenolpyruvate; 138-08-9; 1nhx; 2-(phosphonooxy)-2-propenoic acid; 2-(phosphonooxy)prop-2-enoic acid; 2-Dihydroxyphosphinoyloxyacrylic acid; 2-Phosphonooxyprop-2-enoic acid; 2-Propenoic acid, 2-(phosphonooxy)-; 2-phosphonooxyprop-2-enoate; 545YL308OW; 923-14-8; AC1L1AIQ; Barium silver 2-(phosphonatooxy)acrylate; CHEBI:44897; EINECS 205-312-2; EINECS 213-089-8; PEP; UNII-545YL308OW; bmse000107; DTBNBXWJWCWCIK-UHFFFAOYSA-N
|
| Indication |
Discovery agent
|
Investigative
|
[1]
|
| Structure |
|
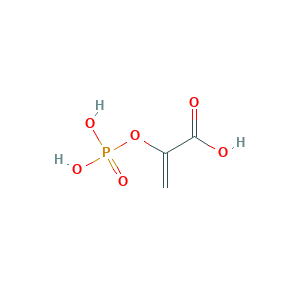 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
168.04 |
Topological Polar Surface Area |
104 |
| Heavy Atom Count |
10 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 1005
- PubChem SID
-
3374
; 583652
; 584923
; 820197
; 820473
; 827615
; 828410
; 828556
; 828558
; 828817
; 854810
; 4790277
; 7889796
; 8009228
; 8009230
; 8009232
; 8013976
; 8020252
; 8020259
; 8025541
; 8150924
; 11538112
; 14772543
; 24277639
; 24439362
; 24697526
; 24697892
; 24775896
; 24874887
; 26697124
; 26717277
; 26737585
; 46392608
; 46506098
; 49681292
; 49703243
; 57320663
; 57410861
; 57578075
; 57654765
; 75356424
; 81064563
; 85164943
; 85856284
; 92298602
; 99239587
; 99239589
; 104251378
; 104296495
; 124361194
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T3AE
- Formula
- C3H5O6P
- Canonical SMILES
- C=C(C(=O)O)OP(=O)(O)O
- InChI
- 1S/C3H5O6P/c1-2(3(4)5)9-10(6,7)8/h1H2,(H,4,5)(H2,6,7,8)
- InChIKey
- DTBNBXWJWCWCIK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


