| Synonyms |
Carubinose; D(+)-Mannose; D-(+)-Mannose; D-Mannopyranose; D-Mannopyranoside; D-Mannose; D-Mannose,(S); DL-Mannose; Mannopyranose; Mannopyranose, D-; Mannopyranoside; Mannose; Seminose; WQZGKKKJIJFFOK-QTVWNMPRSA-N; (+-)-Mannose; (3S,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol; 530-26-7; AC1L2D5C; CHEBI:4208; CHEMBL469448; D-Man; DTXSID5040463; EINECS 208-474-2; Epitope ID:152206; GTPL4650; MLS001332527; MLS001332528; Man; SCHEMBL38300; SMR000857125; alpha,beta-D-mannopyranose; bmse000018; bmse000874; bmse000882
|
| Cross-matching ID |
- PubChem CID
- 18950
- PubChem SID
-
3459
; 824810
; 838927
; 3135279
; 8027205
; 8164149
; 14720290
; 15066909
; 15322061
; 22396012
; 24277482
; 24882738
; 24882739
; 24896601
; 24897068
; 26737907
; 29217866
; 29286577
; 53788969
; 56311267
; 56320690
; 56323500
; 56475044
; 57330235
; 57651949
; 79376412
; 85164870
; 87572161
; 91700886
; 92298697
; 92710545
; 93375117
; 103583168
; 104346557
; 124801461
; 125310805
; 126523054
; 126631771
; 127844171
; 129621449
; 131317572
; 131465283
; 134228441
; 134228449
; 134958993
; 134984714
; 134999617
; 135136220
; 137002180
; 139416018
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0GS9E
- Formula
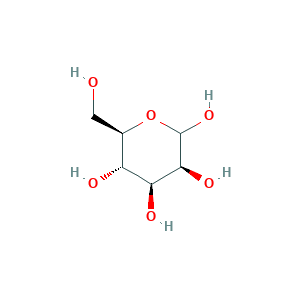
- C6H12O6
- Canonical SMILES
- C(C1C(C(C(C(O1)O)O)O)O)O
- InChI
- 1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5+,6?/m1/s1
- InChIKey
- WQZGKKKJIJFFOK-QTVWNMPRSA-N
|