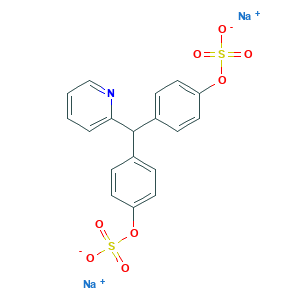
| Synonyms |
Laxidogol; Laxoberal; Laxoberon; Prepopik (TN); LA 391; LA-391; LS-105107; NCGC00182711-01; Natrii picosulfas; Natrii picosulfas [INN-Latin]; Natriumpicosulfat; Natriumpikosulfat; Neopax; 10040-45-6; 2-Picolylidenebis(p-phenyl sodium sulfate); 4,4'-(2-Picolylidene)bis(phenylsulfuric acid) disodium salt; 4,4'-(2-Pyridinylmethylene)bisphenol bis(hydrogen sulfate) (ester) disodium salt; 4,4'-(2-Pyridylmethylene)diphenol bis(hydrogen sulfate) disodium salt; 4,4'-(2-Pyridylmethylene)diphenolbis(hydrogen sulfate) (ester) disodium salt; 4,4'-(2-picolylidene)bisphenylsulfuric acid; 4,4'-(picoliliden)-bis-phenylsulphate; AK546436; AS-15785; Anhydrous Sodium Picosulfate; BCP11522; C-36558; C13072; C18H13NNa2O8S2; CAS-10040-45-6; CHEBI:32147; CHEMBL1697768; CS-2645; DA 1773; DA-1773; DSSTox_CID_28589; DSSTox_GSID_48663; DSSTox_RID_82860; DTXSID7048663; Disodium 4,4'-(2-pyridylmethylene)-di(phenyl sulphate);Disodium 4,4'-(2-pyridylmethylene)-di(phenyl sulphate);Sodium picosulfate; Disodium 4,4'-disulfoxydiphenyl-(2-pyridyl)methane; EINECS 233-120-9; Evacuol; Evanol; FT-0673898; GOZDTZWAMGHLDY-UHFFFAOYSA-L; Guttalax; Guttalax-Fher; HMS3652K17; HY-B0544; KS-00000H2M; Phenol, 4,4'-(2-pyridylmethylene)bis-, bis(hydrogen sulfate), disodium salt; Pico-Salax; Picolax; Picoprep; Picosulfate de sodium; Picosulfate de sodium [INN-French]; Picosulfate sodium; Picosulfate sodium salt; Picosulfato sodico; Picosulfato sodico [INN-Spanish]; Picosulfol; Q-201722; Q410265; Q783; Rapilax; S0936; SCHEMBL346436; SW219183-1; Sodium Picosulfate [INN:BAN:JAN]; Sodium picosulfate hydrate; Sodium picosulphate; Tox21_113026; UNII-VW106606Y8; VW106606Y8; disodium;[4-[pyridin-2-yl-(4-sulfonatooxyphenyl)methyl]phenyl] sulfate; phenol, 4,4'-(2-pyridinylmethylene)bis-, 1,1'-bis(hydrogen sulfate), sodium salt (1:2); s4020; sodium (pyridin-2-ylmethylene)bis(4,1-phenylene) bis(sulfate); sodium picosulfate
|
| Cross-matching ID |
- PubChem CID
- 68654
- PubChem SID
-
585136
; 8192250
; 14810109
; 43125192
; 50006101
; 57316986
; 75609776
; 104342700
; 117374714
; 124976358
; 126671820
; 135027201
; 137118936
; 144206577
; 164178032
; 164232631
; 188889269
; 198975000
; 210279710
; 210282033
; 223441830
; 223652695
; 224488080
; 226681202
; 252356710
; 252543057
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00TGR
- Formula
- C18H13NNa2O8S2
- Canonical SMILES
- C1=CC=NC(=C1)C(C2=CC=C(C=C2)OS(=O)(=O)[O-])C3=CC=C(C=C3)OS(=O)(=O)[O-].[Na+].[Na+]
- InChI
- 1S/C18H15NO8S2.2Na/c20-28(21,22)26-15-8-4-13(5-9-15)18(17-3-1-2-12-19-17)14-6-10-16(11-7-14)27-29(23,24)25;;/h1-12,18H,(H,20,21,22)(H,23,24,25);;/q;2*+1/p-2
- InChIKey
- GOZDTZWAMGHLDY-UHFFFAOYSA-L
|