Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2464) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Dihydrodaidzein
|
|||||
| Synonyms |
BID0517; Dihydro Daidzein; Dihydrodaidzein (keto); Dihydrodazein; Isoflavanone, 4',7-dihydroxy-; JHYXBPPMXZIHKG-UHFFFAOYSA-N; SCHEMBL131543; ZX-AFC001619; dihydrodaidzein; (+/-)-Dihydrodaidzein; (R,S)-2,3-Dihydrodaidzein; 17238-05-0; 2,3-dihydro-7-hydroxy-3-(4-hydroxyphenyl)-4H-1-Benzopyran-4-one; 4',7-dihydroxy-Isoflavanone; 7,4'-Dihydroxyisoflavanone; 7-hydroxy-3-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one; 7-hydroxy-3-(4-hydroxyphenyl)chroman-4-one; CHEBI:75842; CTK8C3008; DTXSID70912308; HY-N1461; SCHEMBL16152440
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
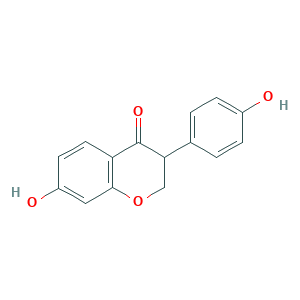 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 256.25 | Topological Polar Surface Area | 66.8 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

