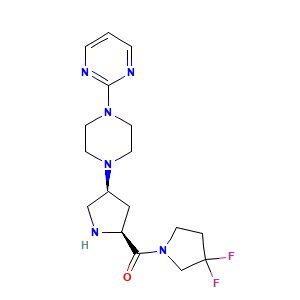
| Synonyms |
GOSOGLIPTIN; 869490-23-3; UNII-GI718UO477; PF-00734200; PF-734200; CHEMBL515387; GI718UO477; 2-(4-{(3s,5s)-5-[(3,3-Difluoropyrrolidin-1-Yl)carbonyl]pyrrolidin-3-Yl}piperazin-1-Yl)pyrimidine; 869490-47-1; (3,3-difluoropyrrolidin-1-yl)-[(2S,4S)-4-(4-pyrimidin-2-ylpiperazin-1-yl)pyrrolidin-2-yl]methanone; (3,3-difluoropyrrolidin-1-yl)((2S,4S)-4-(4-(pyrimidin-2-yl)piperazin-1-yl)pyrrolidin-2-yl)methanone; (3,3-DIFLUOROPYRROLIDIN-1-YL)[(2S,4S)-4-[4-(PYRIMIDIN-2-YL)PIPERAZIN-1-YL]PYRROLIDIN-2-YL]METHANONE; Gosogliptin [USAN:
|
| Cross-matching ID |
- PubChem CID
- 11516136
- CAS Number
-
- TTD Drug ID
- D07HZG
- Formula
- C17H24F2N6O
- Canonical SMILES
- C1CN(CC1(F)F)C(=O)[C@@H]2C[C@@H](CN2)N3CCN(CC3)C4=NC=CC=N4
- InChI
- InChI=1S/C17H24F2N6O/c18-17(19)2-5-25(12-17)15(26)14-10-13(11-22-14)23-6-8-24(9-7-23)16-20-3-1-4-21-16/h1,3-4,13-14,22H,2,5-12H2/t13-,14-/m0/s1
- InChIKey
- QWEWGXUTRTXFRF-KBPBESRZSA-N
|