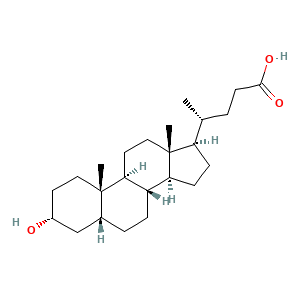
| Synonyms |
Lithocholic acid; LITHOCHOLIC ACID; 434-13-9; Lithocolic acid; Lithocholate; 3alpha-Hydroxy-5beta-cholanic acid; 3alpha-Hydroxy-5beta-cholan-24-oic acid; 3-Hydroxycholan-24-oic acid; 3alpha-Hydroxycholanic acid; 3-alpha-Hydroxycholanic acid; 5beta-Cholanic acid-3alpha-ol; NCI-C03861; 3alpha-Hydroxy-5beta-cholanoic acid; (3alpha,5beta)-3-hydroxycholan-24-oic acid; 3-alpha-Hydroxy-5-beta-cholanic acid; CCRIS 363; UNII-5QU0I8393U; 3alpha-Hydroxy-5beta-cholanate; 5-beta-Cholanic acid, 3-alpha-hydroxy-; Cholan-24-oic acid, 3-hydroxy-,
|
| Cross-matching ID |
- PubChem CID
- 9903
- CAS Number
-
- TTD Drug ID
- D04SLF
- Formula
- C24H40O3
- Canonical SMILES
- C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@H]4[C@@]3(CC[C@H](C4)O)C)C
- InChI
- InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1
- InChIKey
- SMEROWZSTRWXGI-HVATVPOCSA-N
|