| General Information of Drug (ID:
DR4349) |
| Drug Name |
SNX-5422
|
| Prodrug Info |
SNX-5422 is the prodrug of Isonicotinic acyl-NADH
|
| Synonyms |
908115-27-5; PF-04929113; SNX-5422; SNX 5422; SNX5422; UNII-BF52J69Q8T; PF 04929113; PF-04929113 (SNX-5422); BF52J69Q8T; PF04929113; PF-4929113; cc-40; MLS006011083; SCHEMBL1220791; SCHEMBL1220790; CHEMBL1195136; SCHEMBL15604989; DTXSID50238270; C25H30F3N5O4; MolPort-023-293-502; BCPP000065; HMS3656B09; EX-A2343; BCP02427; s2656; ZINC95616595; ABP000737; ZINC100071931; ZINC252517142; AKOS027276395; SB16643; DB06070; API0008143; RL05706; SNX-5422 /; CS-0272; NCGC00346640-01; NCGC00386184-02; Glycine, trans-4-((2-(aminocarbonyl)-5-(4,5,6,7-tetrah
|
| Indication |
Acute lymphoblastic leukemia
[ICD11: 2B33]
|
Phase 1/2
|
[1]
|
|
Lung cancer
[ICD11:
ICD11: 2C25]
|
Phase 1/2
|
[2]
|
|
Chronic lymphocytic leukaemia
[ICD11:
ICD11: 2A82]
|
Phase 1
|
[3]
|
|
Stomach cancer
[ICD11:
ICD11: 2B72]
|
Phase 1
|
[4]
|
| Structure |
|
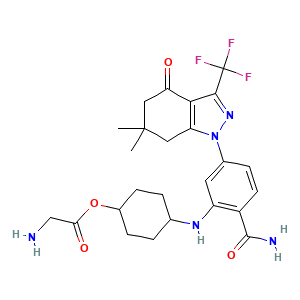 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
521.5 |
Topological Polar Surface Area |
142 |
| Heavy Atom Count |
37 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
10 |
| Cross-matching ID |
- PubChem CID
- 44195571
- CAS Number
-
- TTD Drug ID
- D07MPL
- Formula
- C25H30F3N5O4
- Canonical SMILES
- CC1(CC2=C(C(=O)C1)C(=NN2C3=CC(=C(C=C3)C(=O)N)NC4CCC(CC4)OC(=O)CN)C(F)(F)F)C
- InChI
- InChI=1S/C25H30F3N5O4/c1-24(2)10-18-21(19(34)11-24)22(25(26,27)28)32-33(18)14-5-8-16(23(30)36)17(9-14)31-13-3-6-15(7-4-13)37-20(35)12-29/h5,8-9,13,15,31H,3-4,6-7,10-12,29H2,1-2H3,(H2,30,36)
- InChIKey
- AVDSOVJPJZVBTC-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


