| General Information of Drug (ID:
DR5118) |
| Drug Name |
Acetohydroxamic Acid
|
| Synonyms |
AHA; Acethydroxamsaeure; Acethydroxamsaure; Acetohydroxamate; HAE; Lithostat; Acethydroxamic acid; Acethydroxamsaeure [German]; Acetohydroximic acid; Acetyl hydroxyamino; Acetylhydroxamic acid; Acide acetohydroxamique; Acide acetohydroxamique [French]; Acido acetohidroxamico; Acido acetohidroxamico [Spanish]; Acidum acetohydroxamicum; Acidum acetohydroxamicum [Latin]; Cetohyroxamic acid; Methylhydroxamic acid; SJX HLdmMAH; AHA (TN); Acetic acid, oxime; Acetohydroxamic acid [USAN:INN]; Lithostat (TN); N-Acetyl hydroxyacetamide; N-Acetylhydroxylamine; N-Hydroxyacetamide; N-hydroxyacetimidic acid; N-hydroxyethanimidic acid; S14-0751; Acetohydroxamic acid (USP/INN); Acetamide, N-hydroxy-(9CI)
|
| Indication |
Urinary tract infection
[ICD11: GC08]
|
Approved
|
[1]
|
| Structure |
|
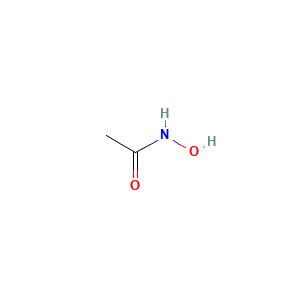 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
75.07 |
Topological Polar Surface Area |
49.3 |
| Heavy Atom Count |
5 |
Rotatable Bond Count |
0 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 1990
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0R9BG
- Formula
- C2H5NO2
- Canonical SMILES
- CC(=O)NO
- InChI
- InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4)
- InChIKey
- RRUDCFGSUDOHDG-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


