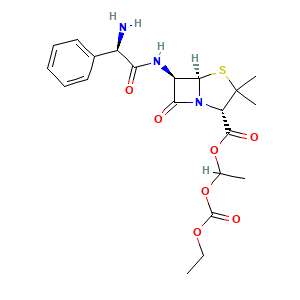
| Synonyms |
Bacampicilina; Bacampicilline; Bacampicillinum; Penglobe; Bacampicillin hydrochloride; Bacampicilina [INN-Spanish]; Bacampicillin (INN); Bacampicillin [INN:BAN]; Bacampicilline [INN-French]; Bacampicillinum [INN-Latin]; Penglobe (TN); Spectrobid (TN); (2S,5R,6R)-6((R)-(2-Amino-2-phenylacetamido))-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid ester with ethyl 1-hydroxyethylcarbonate; 1'-Ethoxycarbonyloxyethyl-(6-D-alpha-aminophenylacetamido)penicillanate; 1-Ethoxycarbonyloxyethyl (2S,5R,6R)-6((R)-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptan-2-carboxylat; 1-[(ethoxycarbonyl)oxy]ethyl (2S,5R,6R)-6-{[(2R)-2-amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-[(ethoxycarbonyl)oxy]ethyl 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R,6R)-6-[[(2S)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 6-((R)-2-Amino-2-phenylacetamido)penicillansaeure-(1-(ethoxycarbonyloxy)ethylester
|
| Cross-matching ID |
- PubChem CID
- 441397
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04CFW
- Formula
- C21H27N3O7S
- Canonical SMILES
- CCOC(=O)OC(C)OC(=O)[C@H]1C(S[C@H]2N1C(=O)[C@H]2NC(=O)[C@@H](C3=CC=CC=C3)N)(C)C
- InChI
- InChI=1S/C21H27N3O7S/c1-5-29-20(28)31-11(2)30-19(27)15-21(3,4)32-18-14(17(26)24(15)18)23-16(25)13(22)12-9-7-6-8-10-12/h6-11,13-15,18H,5,22H2,1-4H3,(H,23,25)/t11?,13-,14-,15+,18-/m1/s1
- InChIKey
- PFOLLRNADZZWEX-FFGRCDKISA-N
|