| Synonyms |
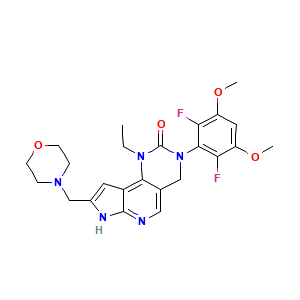
Unii-Y6BX7BL23K; UNII-Y6BX7BL23K; Y6BX7BL23K; GTPL9767; SCHEMBL15556271; HCDMJFOHIXMBOV-UHFFFAOYSA-N; example 126 [WO2014007951]; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-4,7-dihydropyrrolo[4,5]pyrido[1,2-d]pyrimidin-2-one; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d]pyrimidin-2-one; INCB54828
|
| Cross-matching ID |
- PubChem CID
- 86705695
- CAS Number
-
- TTD Drug ID
- D0O6UY
- Formula
- C24H27F2N5O4
- Canonical SMILES
- CCN1C2=C3C=C(NC3=NC=C2CN(C1=O)C4=C(C(=CC(=C4F)OC)OC)F)CN5CCOCC5
- InChI
- InChI=1S/C24H27F2N5O4/c1-4-30-21-14(11-27-23-16(21)9-15(28-23)13-29-5-7-35-8-6-29)12-31(24(30)32)22-19(25)17(33-2)10-18(34-3)20(22)26/h9-11H,4-8,12-13H2,1-3H3,(H,27,28)
- InChIKey
- HCDMJFOHIXMBOV-UHFFFAOYSA-N
|