Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0006) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Naphthoquinone beta
|
|||||
| Synonyms |
Beta-Naphthoquinone; Naphthalene-1,2-dione; 1,2-Naphthoquinone; o-Naphthoquinone; KETQAJRQOHHATG-UHFFFAOYSA-N; NAPHTHALENEDIONE; .beta.-Naphthoquinone; 1,2-Dihydro-1,2-diketo-naphthalene; 1,2-NAPHTHOQUINONE; 1,2-Naftochinon; 1,2-Naftochinon [Czech]; 1,2-Naphthalenedione; 1,2-Naphthaquinone; 1,2-Naphthoquinone, 95%, tech.; 1,2-dihydronaphthalene-1,2-dione; 524-42-5; 804K62F61Q; AI3-14930; BRN 0606546; C10H6O2; CCRIS 1558; CHEBI:34055; CHEMBL52347; EINECS 208-360-2; HSDB 2036; MFCD00001698; MLS000069467; NSC 9831; SMR000059112; UNII-804K62F61Q
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
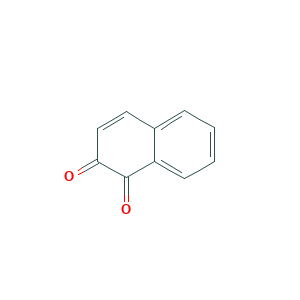 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 158.15 | Topological Polar Surface Area | 34.1 | ||
| Heavy Atom Count | 12 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

