Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0015) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Nitrophenyl acetate
|
|||||
| Synonyms |
Aceticacid,P-Nitrophenylester; 4-Nitrophenyl acetate; P-NITROPHENYL ACETATE; QAUUDNIGJSLPSX-UHFFFAOYSA-N; p-Acetoxynitrobenzene; p-Nitrobenzene acetate; p-Nitrophenol acetate; p-Nitrophenyl acetate (VAN); para-nitrophenyl acetate; (4-nitrophenyl) acetate; 4-Nitrophenyl acetate, 97%; 830-03-5; AI3-00496; Acetic acid 4-nitrophenyl ester; Acetic acid p-nitrophenyl ester; Acetic acid, 4-nitrophenyl ester; Acetic acid, p-nitrophenyl ester; BRN 0515874; CHEBI:82635; EINECS 212-593-5; I902J0QH9S; MFCD00007326; NSC 2633; UNII-I902J0QH9S
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
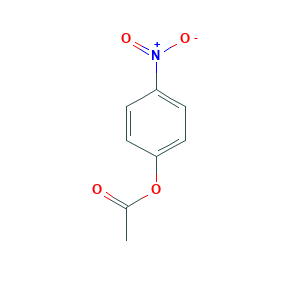 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 181.15 | Topological Polar Surface Area | 72.1 | ||
| Heavy Atom Count | 13 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

