| Synonyms |
Aclometasone; A-Chloro-16; A-methyl Prednisolone; A-methylprednisolone; Alclometasone (INN); Alclometasone [INN:BAN]; Alclomethasone; SCHEMBL4127; ZINC30691420; alclometasone; (7alpha,11beta,16alpha)-7-chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 136H45TB7B; 67452-97-5; 7alpha-Chloro-16alpha-methylprednisolone; AC1NSJSZ; CHEBI:53776; CHEMBL1201361; DB00240; UNII-136H45TB7B
|
| Cross-matching ID |
- PubChem CID
- 5311000
- PubChem SID
-
7978656
; 11056200
; 15451271
; 39340733
; 46508296
; 51071561
; 51091455
; 87246635
; 114155019
; 124963548
; 135199337
; 135834189
; 137019440
; 137234003
; 141792513
; 162258587
; 164838234
; 175265310
; 180371900
; 226396178
; 252562151
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0F1EX
- Formula
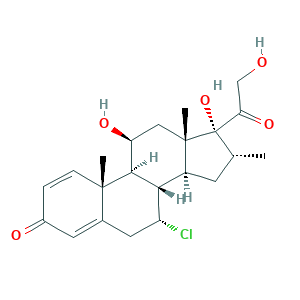
- C22H29ClO5
- Canonical SMILES
- CC1CC2C3C(CC4=CC(=O)C=CC4(C3C(CC2(C1(C(=O)CO)O)C)O)C)Cl
- InChI
- 1S/C22H29ClO5/c1-11-6-14-18-15(23)8-12-7-13(25)4-5-20(12,2)19(18)16(26)9-21(14,3)22(11,28)17(27)10-24/h4-5,7,11,14-16,18-19,24,26,28H,6,8-10H2,1-3H3/t11-,14+,15-,16+,18-,19+,20+,21+,22+/m1/s1
- InChIKey
- FJXOGVLKCZQRDN-PHCHRAKRSA-N
|