| General Information of Drug (ID:
DR0076) |
| Drug Name |
Alprenolol
|
| Synonyms |
Alfeprol; Alfeprol [Russian]; Alpheprol; Alprenolol [INN:BAN]; Alprenololum; Alprenololum [INN-Latin]; PAZJSJFMUHDSTF-UHFFFAOYSA-N; alprenolol; dl-Alprenolol; 1-(2-Allylphenoxy)-3-isopropylamino-2-propanol; 1-(o-Allylphenoxy)-3-(isopropylamino)-2-propanol; 13655-52-2; 2-Propanol, 1-((1-methylethyl)amino)-3-(2-(2-propenyl)phenoxy)-; 2-Propanol, 1-(o-allylphenoxy)-3-(isopropylamino)-; 2-Propanol, 1-[(1-methylethyl)amino]-3-[2-(2-propenyl)phenoxy]-; CHEBI:51211; CHEMBL266195; EINECS 237-140-9; NCGC00015099-05; Yobir
|
| Indication |
Essential hypertension
[ICD11: BA00]
|
Phase 4
|
[1]
|
| Structure |
|
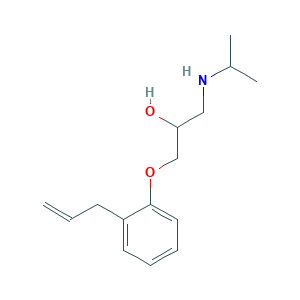 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
249.35 |
Topological Polar Surface Area |
41.5 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 2119
- PubChem SID
-
7403854
; 8151437
; 10507180
; 11335190
; 11360429
; 11363062
; 11365624
; 11368186
; 11371971
; 11374790
; 11376348
; 11461401
; 11466278
; 11467398
; 11485563
; 11485995
; 11489630
; 11490881
; 11493030
; 11493982
; 11534311
; 15044941
; 26752306
; 29221299
; 46506033
; 47290959
; 47440065
; 47736281
; 47810579
; 47810580
; 47885235
; 48034925
; 48034926
; 48184817
; 49698847
; 50022618
; 50105225
; 50105226
; 51091495
; 56413052
; 57321152
; 80044758
; 85209662
; 85788463
; 90340777
; 92308802
; 96099927
; 103164453
; 103852444
; 104171111
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01VAB
- Formula
- C15H23NO2
- Canonical SMILES
- CC(C)NCC(COC1=CC=CC=C1CC=C)O
- InChI
- 1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3
- InChIKey
- PAZJSJFMUHDSTF-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


