| Synonyms |
Amcinonido; Amcinonido [INN-Spanish]; Amcinonidum; Amcinonidum [INN-Latin]; Cyclocort; Cyclocort (TN); Triamcinolonacetatcyclopentanonid; Visderm; amcinonida; amcinonide; 19alpha-Fluor-11beta,21-dihydroxy-16alpha,17alpha-(tetramethylen)methylendioxy-1,4-pregnadien-3,20-dion 21-acetat; 423W026MA9; 51022-69-6; C28H35FO7; CHEBI:31199; CL 34699; CL-34699; EINECS 256-915-2; MLS000028656; MLS001333715; SMR000058920; UNII-423W026MA9
|
| Cross-matching ID |
- PubChem CID
- 443958
- PubChem SID
-
855515
; 7848450
; 7978681
; 10299444
; 15084976
; 24890657
; 36887100
; 46506705
; 50926096
; 56352910
; 56422434
; 56423154
; 57404572
; 57653835
; 71825482
; 92712334
; 93576974
; 99368349
; 103770715
; 104631033
; 124801285
; 126625654
; 126656779
; 134338306
; 135001553
; 135653543
; 136014083
; 137005249
; 138911215
; 139999915
; 144204964
; 152100586
; 160963636
; 162198485
; 175266120
; 175612165
; 178103639
; 179116554
; 226396660
; 241090273
; 250133950
; 252401183
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06XHC
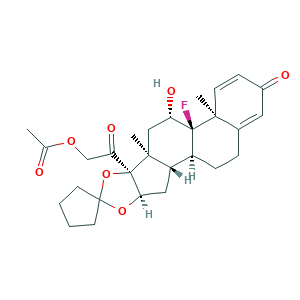
- Formula
- C28H35FO7
- Canonical SMILES
- CC(=O)OCC(=O)C12C(CC3C1(CC(C4(C3CCC5=CC(=O)C=CC54C)F)O)C)OC6(O2)CCCC6
- InChI
- 1S/C28H35FO7/c1-16(30)34-15-22(33)28-23(35-26(36-28)9-4-5-10-26)13-20-19-7-6-17-12-18(31)8-11-24(17,2)27(19,29)21(32)14-25(20,28)3/h8,11-12,19-21,23,32H,4-7,9-10,13-15H2,1-3H3/t19-,20-,21-,23+,24-,25-,27-,28+/m0/s1
- InChIKey
- ILKJAFIWWBXGDU-MOGDOJJUSA-N
|