| General Information of Drug (ID:
DR0109) |
| Drug Name |
Amsacrine
|
| Synonyms |
Acridinyl Anisidide; Acridinylanisidide; Amekrin; Amsacrina; Amsacrina [INN-Spanish]; Amsacrinum; Amsacrinum [INN-Latin]; Amsidine; Amsidyl; Amsine; Lamasine; SN 21429; SN-11841; amsacrine; m-AMSA; meta-Amsacrine; 4'-(9-Acridinylamino)-3'-methoxymethanesulfonanilide; 4'-(9-Acridinylamino)methanesulfon-m-anisidide; 4'-(9-Acridinylamino)methanesulphon-m-anisidide; 51264-14-3; AMSA; C21H19N3O3S; CI-880; N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide; NSC 156303; NSC 249992; NSC-249992; NSC249992; UNII-00DPD30SOY; mAMSA
|
| Indication |
Acute lymphoblastic leukemia
[ICD11: 2B33]
|
Phase 4
|
[1]
|
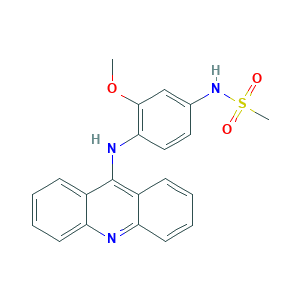
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
393.5 |
Topological Polar Surface Area |
88.7 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 2179
- PubChem SID
-
4711
; 137181
; 597748
; 5128698
; 7849380
; 7978704
; 8137254
; 8151483
; 11110803
; 11406388
; 11447254
; 11533500
; 12013305
; 14927834
; 29221358
; 46507539
; 47440375
; 48110554
; 48415564
; 48422855
; 49815687
; 49855467
; 50110958
; 53789073
; 57321186
; 78687979
; 90341308
; 99453443
; 103166066
; 103919715
; 103925608
; 104299838
; 118043462
; 121379628
; 124633410
; 124879323
; 124879324
; 125824264
; 126659536
; 126687025
; 127318462
; 127318463
; 127318464
; 127318465
; 127318466
; 127318467
; 127318468
; 127318469
; 127318470
; 127318471
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G9YH
- Formula
- C21H19N3O3S
- Canonical SMILES
- COC1=C(C=CC(=C1)NS(=O)(=O)C)NC2=C3C=CC=CC3=NC4=CC=CC=C42
- InChI
- 1S/C21H19N3O3S/c1-27-20-13-14(24-28(2,25)26)11-12-19(20)23-21-15-7-3-5-9-17(15)22-18-10-6-4-8-16(18)21/h3-13,24H,1-2H3,(H,22,23)
- InChIKey
- XCPGHVQEEXUHNC-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


