Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0110) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
AI3-25184
|
|||||
| Synonyms |
Amyl nitrite; KSC492M8F; N-Phenyl nitrile; Nitramyl; Nitrous acid, pentyl ester; Nitrousacid, pentyl ester; Pentyl alcohol, nitrite; Pentyl nitrite; Pentyl nitrite, AldrichCPR; Pentylnitrite; UN 1113 (Related); amyl 1 nitrite; n-Amyl nitrite; n-Pentyl nitrite; n-pentylnitrite; nitrous acid pentyl ester; 463-04-7; AC1L1UC5; ACMC-1ACPW; AI3-25184; BRN 1701241; CCRIS 763; CHEBI:55344; CSDTZUBPSYWZDX-UHFFFAOYSA-N; DSSTox_CID_4522; DSSTox_GSID_24522; DSSTox_RID_77442; EINECS 207-332-7; H2HUX79FYK; SCHEMBL34065; UNII-H2HUX79FYK
|
|||||
| Indication | Achalasia [ICD11: DA21] | Phase 1 | [1] | |||
| Structure |
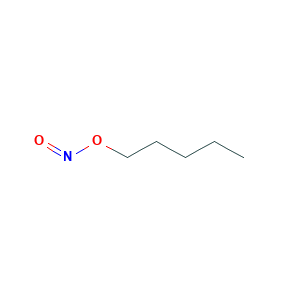 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 117.15 | Topological Polar Surface Area | 38.7 | ||
| Heavy Atom Count | 8 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

