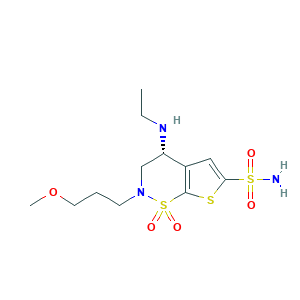
| Synonyms |
Birnzolamide; Brinzolamide; (4R)-4-(ethylamino)-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide; (R)-4-(Ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2H-thieno(3,2-e)-1,2-thiazine-6-sulfonamide 1,1-dioxide; (R)-4-(Ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide; 138890-62-7; AL 4862; AL-4862; Azopt; C12H21N3O5S3; CHEBI:3176; MFCD08067749; UNII-9451Z89515
|
| Cross-matching ID |
- PubChem CID
- 68844
- PubChem SID
-
9962
; 7847718
; 7886394
; 8192384
; 11466393
; 11467513
; 11486146
; 11528766
; 12015162
; 14780300
; 14878101
; 26718765
; 29214990
; 43125358
; 46394376
; 46507071
; 46511455
; 47871124
; 47871125
; 48020430
; 48395296
; 49699073
; 56463043
; 57317102
; 74925916
; 85788886
; 87325228
; 92125455
; 92721410
; 92729740
; 93166197
; 103914463
; 104234232
; 104343207
; 109692908
; 117630731
; 118048468
; 121363126
; 124800916
; 126627643
; 126659540
; 126661718
; 128041764
; 134337893
; 135028809
; 135916574
; 137005796
; 143102638
; 144204273
; 152159683
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01MUR
- Formula
- C12H21N3O5S3
- Canonical SMILES
- CCNC1CN(S(=O)(=O)C2=C1C=C(S2)S(=O)(=O)N)CCCOC
- InChI
- 1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1
- InChIKey
- HCRKCZRJWPKOAR-JTQLQIEISA-N
|