Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0284) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Cefalexin
|
|||||
| Synonyms |
Carnosporin; Cefablan; Cefadin; Cefadina; Cefaleksin; Cefalexin; Cefalexina; Cefalexine; Cefalexinum; Cefalin; Cefaloto; Cefaseptin; Ceforal; Cefovit; Celexin; Cepastar; Cepexin; Cephacillin; Cephalexin monohydrate; Cephalexine; Cephalexinum; Cephanasten; Ceporex; Ceporexin; Ceporexine; Cophalexin; Factagard; Kefalospes; Keflet; Keflex; Alcephin; Kefolan; Kidolex; Lenocef; Lexibiotico; Medoxine; Novolexin; Ortisporina; Palitrex; Sartosona; Sencephalin; Servispor; Sinthecillin; Sporicef; Tepaxin; Tokiolexin; Zozarine; cephalexin; 15686-71-2; Pectril
|
|||||
| Indication | Acute otitis media [ICD11: AB00] | Approved | [1] | |||
| Structure |
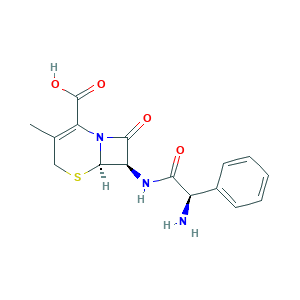 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 347.4 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

