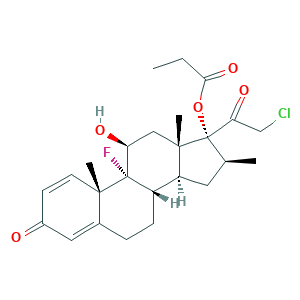
| Synonyms |
Clobestasol propionate; Clobetasol 17-propionate; Clobetasol propionate [USAN:JAN]; Clobex; Cormax; Dermovate; EINECS 246-634-3; Embeline; Embeline E; GR 2/925; MLS000028708; Olux; Olux-E; Temovate; Temovate E; UNII-779619577M; clobetasol 17-propanoate; 21-Chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17-propionate; 25122-46-7; C25H32ClFO5; CCI 4725; CGP 9555; CLOBETASOL PROPIONATE; Clobesol
|
| Cross-matching ID |
- PubChem CID
- 32798
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0H0YD
- Formula
- C25H32ClFO5
- Canonical SMILES
- CCC(=O)OC1(C(CC2C1(CC(C3(C2CCC4=CC(=O)C=CC43C)F)O)C)C)C(=O)CCl
- InChI
- 1S/C25H32ClFO5/c1-5-21(31)32-25(20(30)13-26)14(2)10-18-17-7-6-15-11-16(28)8-9-22(15,3)24(17,27)19(29)12-23(18,25)4/h8-9,11,14,17-19,29H,5-7,10,12-13H2,1-4H3/t14-,17-,18-,19-,22-,23-,24-,25-/m0/s1
- InChIKey
- CBGUOGMQLZIXBE-XGQKBEPLSA-N
|