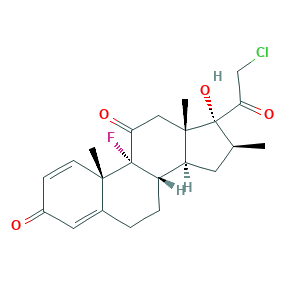
| Synonyms |
Clobetasona; Clobetasona [INN-Spanish]; Clobetasone; Clobetasone (INN); Clobetasone [INN:BAN]; Clobetasonum; Clobetasonum [INN-Latin]; LT69WY1J6D; SCHEMBL3581; XXIFVOHLGBURIG-OZCCCYNHSA-N; ZINC5752185; (8S,9R,10S,13S,14S,16S,17R)-17-(2-chloroacetyl)-9-fluoro-17-hydroxy-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthrene-3,11-dione; 21-Chlor-9-fluor-17-hydroxy-16beta-methyl-1,4-pregnadien-3,11,20-trion; 54063-32-0; AC1L2G2H; API0002061; D07717; DB13158; EINECS 258-953-5; GTPL9088; UNII-LT69WY1J6D
|
| Cross-matching ID |
- PubChem CID
- 71387
- CAS Number
-
- Formula
- C22H26ClFO4
- Canonical SMILES
- CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(=O)CC2(C1(C(=O)CCl)O)C)F)C
- InChI
- 1S/C22H26ClFO4/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,24)17(26)10-20(16,3)22(12,28)18(27)11-23/h6-7,9,12,15-16,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,19-,20-,21-,22-/m0/s1
- InChIKey
- XXIFVOHLGBURIG-OZCCCYNHSA-N
|