| Cross-matching ID |
- PubChem CID
- 5311066
- PubChem SID
-
7979048
; 11056247
; 14855707
; 17397789
; 39340794
; 46506186
; 49834374
; 57359343
; 71840210
; 74651042
; 85403370
; 103771092
; 104179089
; 104253319
; 124757438
; 125164242
; 134338433
; 134975842
; 135653473
; 135692232
; 136311366
; 137125311
; 139999928
; 144206156
; 160964591
; 162178659
; 170464832
; 175268455
; 175612227
; 176484695
; 178103645
; 179150829
; 210279389
; 210281712
; 224521289
; 226395768
; 251915933
; 251917281
; 252347928
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02JNM
- Formula
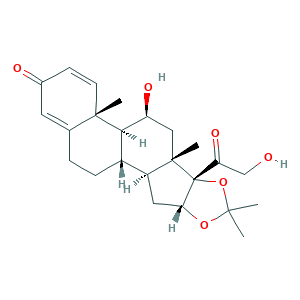
- C24H32O6
- Canonical SMILES
- CC1(OC2CC3C4CCC5=CC(=O)C=CC5(C4C(CC3(C2(O1)C(=O)CO)C)O)C)C
- InChI
- 1S/C24H32O6/c1-21(2)29-19-10-16-15-6-5-13-9-14(26)7-8-22(13,3)20(15)17(27)11-23(16,4)24(19,30-21)18(28)12-25/h7-9,15-17,19-20,25,27H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,20+,22-,23-,24+/m0/s1
- InChIKey
- WBGKWQHBNHJJPZ-LECWWXJVSA-N
|