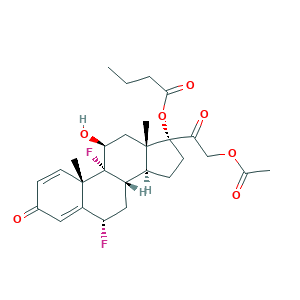
| Synonyms |
Difluprednato; Difluprednato [INN-Spanish]; Difluprednatum; CM 9155; Difluprednate [USAN:INN:JAN]; Difluprednatum [INN-Latin]; Durezol; Epitopic; S8A06QG2QE; W-6309; difluprednate; 23674-86-4; C27H34F2O7; CHEBI:31485; DFBA; Difluoroprednisolone butyrate acetate; MFCD00214273; MLS000028663; MLS001148580; Myser; SMR000058924; UNII-S8A06QG2QE; [(6S,8S,9R,10S,11S,13S,14S,17R)-17-(2-acetyloxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] butanoate
|
| Cross-matching ID |
- PubChem CID
- 443936
- PubChem SID
-
583085
; 856020
; 7848329
; 7979081
; 10299429
; 14811237
; 16650119
; 24894018
; 36887084
; 51530976
; 56422387
; 56423155
; 57404556
; 71840218
; 91703898
; 99443292
; 103771485
; 104630981
; 124799718
; 134995746
; 137239501
; 139999922
; 144206181
; 160967909
; 162184652
; 163835796
; 164178105
; 165235879
; 175268498
; 179150205
; 187051777
; 210275696
; 210281355
; 223441703
; 226396555
; 252359500
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01ZOG
- Formula
- C27H34F2O7
- Canonical SMILES
- CCCC(=O)OC1(CCC2C1(CC(C3(C2CC(C4=CC(=O)C=CC43C)F)F)O)C)C(=O)COC(=O)C
- InChI
- 1S/C27H34F2O7/c1-5-6-23(34)36-26(22(33)14-35-15(2)30)10-8-17-18-12-20(28)19-11-16(31)7-9-24(19,3)27(18,29)21(32)13-25(17,26)4/h7,9,11,17-18,20-21,32H,5-6,8,10,12-14H2,1-4H3/t17-,18-,20-,21-,24-,25-,26-,27-/m0/s1
- InChIKey
- WYQPLTPSGFELIB-JTQPXKBDSA-N
|